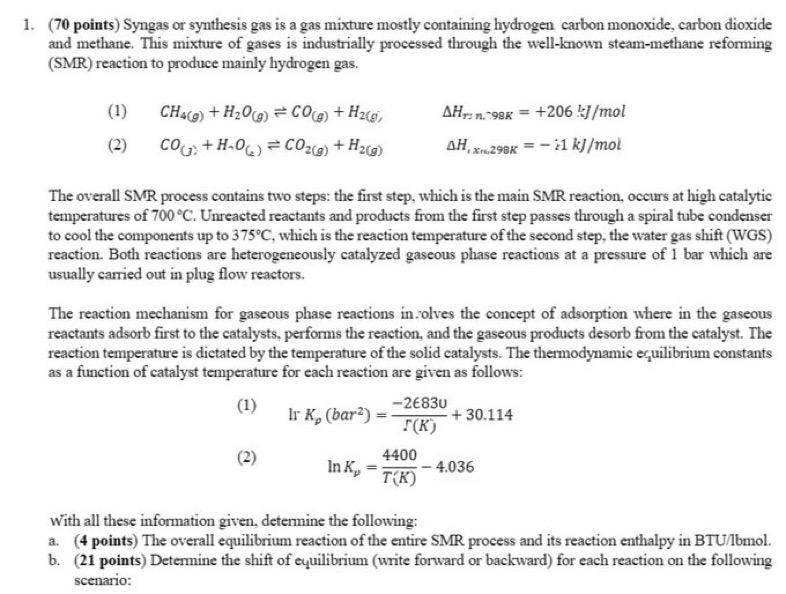
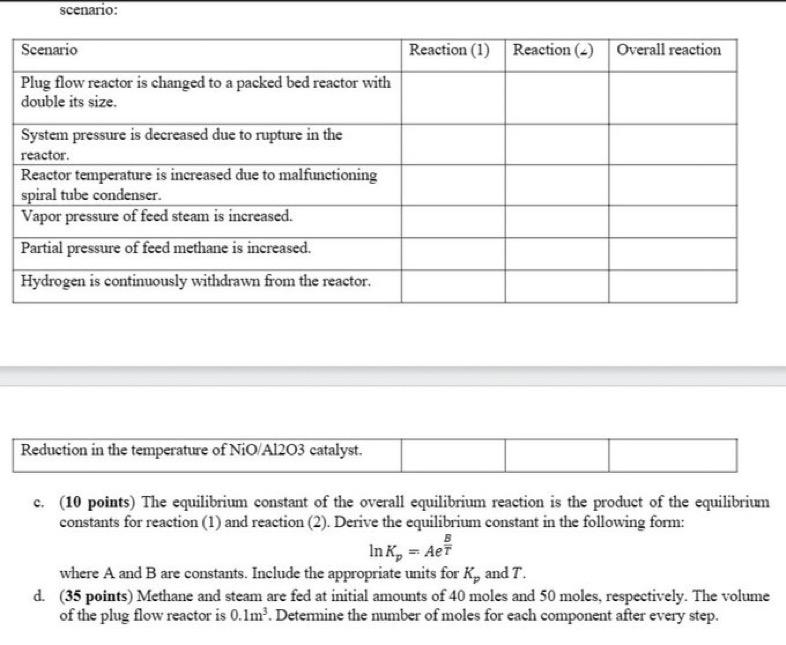
(70 points) Syngas or synthesis gas is a gas mixture mostly containing hydrogen carbon monoxide, carbon dioxide and methane. This mixture of gases is industrially processed through the well-known steam-methane reforming (SMR) reaction to produce mainly hydrogen gas. (1) CH4(g)+H2O(g)CO(g)+H2(g,Hr,n98K=+206kJ/mol (2) CO(g)+HO(l)CO2(g)+H2(g)H,,xn,2,29K=i1kJ/mol The overall SMR process contains two steps: the first step, which is the main SMR reaction, occurs at high catalytic temperatures of 700C. Unreacted reactants and products from the first step passes through a spiral tube condenser to cool the components up to 375C, which is the reaction temperature of the second step, the water gas shift (WGS) reaction. Both reactions are heterogeneously catalyzed gaseous phase reactions at a pressure of 1 bar which are usually carried out in plug flow reactors. The reaction mechanism for gaseous phase reactions in .olves the concept of adsorption where in the gaseous reactants adsorb first to the catalysts, performs the reaction, and the gaseous products desorb from the catalyst. The reaction temperature is dietated by the temperature of the solid catalysts. The thermodynamic ecuilibrium constants as a function of catalyst temperature for each reaction are given as follows: (1) Ir Kp(bar2)=(K)2830+30.114 (2) lnK=T(K)44004.036 With all these information given, determine the following: a. (4 points) The overall equilibrium reaction of the entire SMR process and its reaction enthalpy in BTU/bmol. b. (21 points) Determine the shift of equilibrium (write forward or backward) for each reaction on the following scenario: scenario: c. (10 points) The equilibrium constant of the overall equilibrium reaction is the product of the equilibrium constants for reaction (1) and reaction (2). Derive the equilibrium constant in the following form: lnKp=AeTB where A and B are constants. Include the appropriate units for Kp and T. d. (35 points) Methane and steam are fed at initial amounts of 40 moles and 50 moles, respectively. The volume of the plug flow reactor is 0.1m3. Detemine the number of moles for each component after every step. (70 points) Syngas or synthesis gas is a gas mixture mostly containing hydrogen carbon monoxide, carbon dioxide and methane. This mixture of gases is industrially processed through the well-known steam-methane reforming (SMR) reaction to produce mainly hydrogen gas. (1) CH4(g)+H2O(g)CO(g)+H2(g,Hr,n98K=+206kJ/mol (2) CO(g)+HO(l)CO2(g)+H2(g)H,,xn,2,29K=i1kJ/mol The overall SMR process contains two steps: the first step, which is the main SMR reaction, occurs at high catalytic temperatures of 700C. Unreacted reactants and products from the first step passes through a spiral tube condenser to cool the components up to 375C, which is the reaction temperature of the second step, the water gas shift (WGS) reaction. Both reactions are heterogeneously catalyzed gaseous phase reactions at a pressure of 1 bar which are usually carried out in plug flow reactors. The reaction mechanism for gaseous phase reactions in .olves the concept of adsorption where in the gaseous reactants adsorb first to the catalysts, performs the reaction, and the gaseous products desorb from the catalyst. The reaction temperature is dietated by the temperature of the solid catalysts. The thermodynamic ecuilibrium constants as a function of catalyst temperature for each reaction are given as follows: (1) Ir Kp(bar2)=(K)2830+30.114 (2) lnK=T(K)44004.036 With all these information given, determine the following: a. (4 points) The overall equilibrium reaction of the entire SMR process and its reaction enthalpy in BTU/bmol. b. (21 points) Determine the shift of equilibrium (write forward or backward) for each reaction on the following scenario: scenario: c. (10 points) The equilibrium constant of the overall equilibrium reaction is the product of the equilibrium constants for reaction (1) and reaction (2). Derive the equilibrium constant in the following form: lnKp=AeTB where A and B are constants. Include the appropriate units for Kp and T. d. (35 points) Methane and steam are fed at initial amounts of 40 moles and 50 moles, respectively. The volume of the plug flow reactor is 0.1m3. Detemine the number of moles for each component after every step








