Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7A.Complete the table below for the addition of 1.00 mL of 0.60M HCI to 50.0 mL of the sulfate buffer. Note: complete and submit
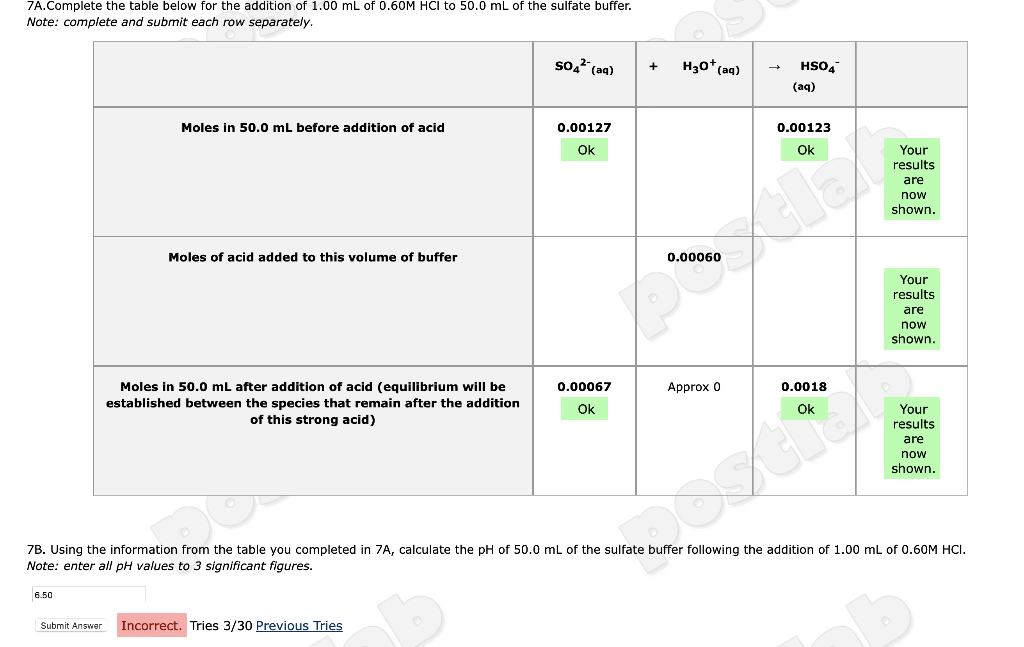
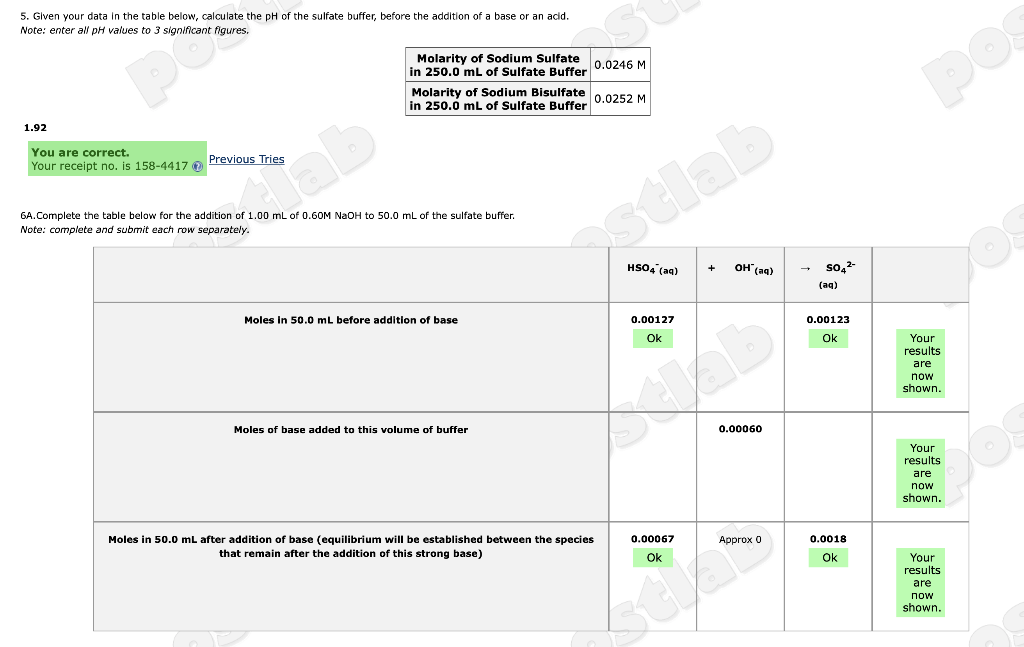
7A.Complete the table below for the addition of 1.00 mL of 0.60M HCI to 50.0 mL of the sulfate buffer. Note: complete and submit each row separately. 6.50 Moles in 50.0 mL before addition of acid Submit Answer Moles of acid added to this volume of buffer Moles in 50.0 mL after addition of acid (equilibrium will be established between the species that remain after the addition of this strong acid) SO4 (aq) Incorrect. Tries 3/30 Previous Tries 0.00127 Ok 0.00067 Ok + H3O+ (aq) 7B. Using the information from the table you completed in 7A, calculate the pH of 50.0 mL of the sulfate buffer following the addition of 1.00 mL of 0.60M HCI. Note: enter all pH values to 3 significant figures. Approx 0 5. Given your data in the table below, calculate the pH of the sulfate buffer, before the addition of a base or an acid. Note: enter all pH values to 3 significant figures. 1.92 You are correct. Your receipt no. is 158-4417 Previous Tries ab Molarity of Sodium Sulfate 0.0246 M in 250.0 mL of Sulfate Buffer Molarity of Sodium Bisulfate in 250.0 mL of Sulfate Buffer 6A. Complete the table below for the addition of 1.00 mL of 0.60M NaOH to 50.0 mL of the sulfate buffer. Note: complete and submit each row separately. Moles in 50.0 mL before addition of base Moles of base added to this volume of buffer 0.0252 M stlab Moles in 50.0 mL after addition f base (equilibrium will be established between the species that remain after the addition of this strong base) HSO4 (aq) 0.00127 Ok 0.00067 Ok + OH(aq) 0.00060 Approx 0 SO4- (aq) 0.00123 Ok 0.0018 Ok Your results are now shown. Your results are now shown. Your results are now shown.
Step by Step Solution
★★★★★
3.49 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
2 Soy act as weak acid sou is conjugate base of weak ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


