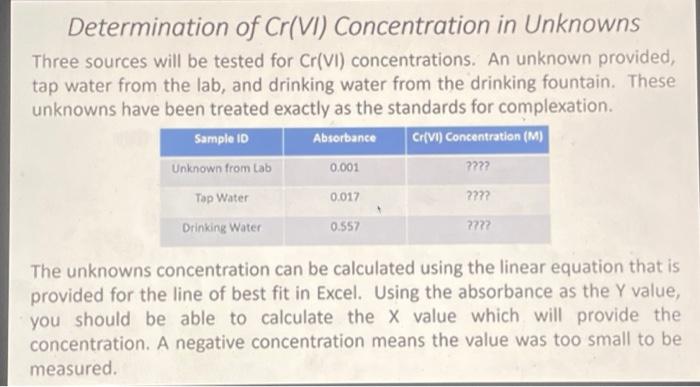
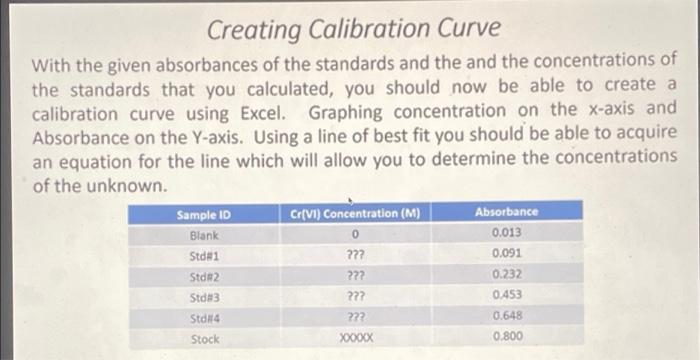
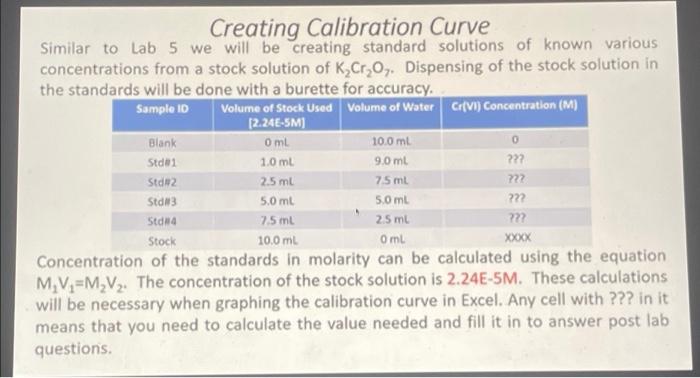
8 15 points Calculate the concentration of the 'Unknown' in ppm (mg/L) of Cr(VI) assuming the source of the chromium is potassium chromate,K2Cros Note: K2Cr20, was used for making the calibration curve. O 276x 105 0.77 1.38 x 105 O 1.44 Determination of Cr(VI) Concentration in Unknowns Three sources will be tested for Cr(VI) concentrations. An unknown provided, tap water from the lab, and drinking water from the drinking fountain. These unknowns have been treated exactly as the standards for complexation. Sample 10 Absorbance Cr(VI) Concentration (M) Unknown from Lab 0.001 ???? Top Water ???? Drinking Water 7772 0.017 0.557 The unknowns concentration can be calculated using the linear equation that is provided for the line of best fit in Excel. Using the absorbance as the Y value, you should be able to calculate the X value which will provide the concentration. A negative concentration means the value was too small to be measured. Creating Calibration Curve With the given absorbances of the standards and the and the concentrations of the standards that you calculated, you should now be able to create a calibration curve using Excel. Graphing concentration on the x-axis and Absorbance on the Y-axis. Using a line of best fit you should be able to acquire an equation for the line which will allow you to determine the concentrations of the unknown. Sample ID Cr(VI) Concentration (M) Absorbance Blank 0.013 ??? Std 2 0.232 0.453 0 Std 1 0.091 222 Std3 222 222 Std 4 Stock 0.648 0.800 Xoooxx Creating Calibration Curve Similar to Lab 5 we will be creating standard solutions of known various concentrations from a stock solution of K Cr,0,. Dispensing of the stock solution in the standards will be done with a burette for accuracy Sample ID Volume of Stock Used Volume of Water Cr(VI) Concentration (M) [2.24E-SM) Blank Om 100 ml Stdei 9.0 mL ??? 1.0 ml 2.5 ml 7.5 ml 722 Std2 Std 3 Stdna 5.0 ml 222 25 ml 22 Stock XXXX 5.0 ml 7.5 mL 10.0 mL Oml Concentration of the standards in molarity can be calculated using the equation M. V=M_V2. The concentration of the stock solution is 2.24E-5M. These calculations will be necessary when graphing the calibration curve in Excel. Any cell with ??? in it means that you need to calculate the value needed and fill it in to answer post lab questions. 11 1 po In terms of its chromium (1) concentration, do you think the "Unknown" sample is suitable for drinking in accordance to the EPA health assessment limitation? Cannot say Yes No