Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8. 9. The ionization enthalpy of Na+ formation from Na(g) is 495.8 kJ mol, while the electron gain enthalpy of Br is - 325.0

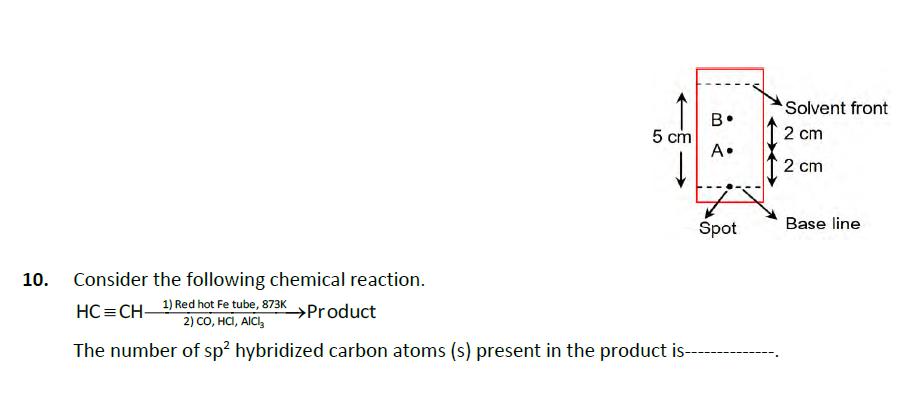
8. 9. The ionization enthalpy of Na+ formation from Na(g) is 495.8 kJ mol, while the electron gain enthalpy of Br is - 325.0 kJ mol-1. Given the lattice enthalpy of NaBr is - 728.4 kJ mol-. The energy for the formation of NaBr ionic solid is (-)- x10kJ mol-1. Using the provided information in the following paper chromatogram: Fig: Paper chromatography for compounds A and B. The calculated R value of A------------ 10-. Solvent front B. 5 cm 2 cm A. 2 cm Consider the following chemical reaction. HC=CH- 1) Red hot Fe tube, 873K >Product 2) CO, HCI, AICI The number of sp hybridized carbon atoms (s) present in the product is--- Spot Base line
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


