Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. The reaction of cyanamide, NHCN(s) with oxygen was run in a bomb calorimeter and AU was found to be -742.24 kJ mol. The
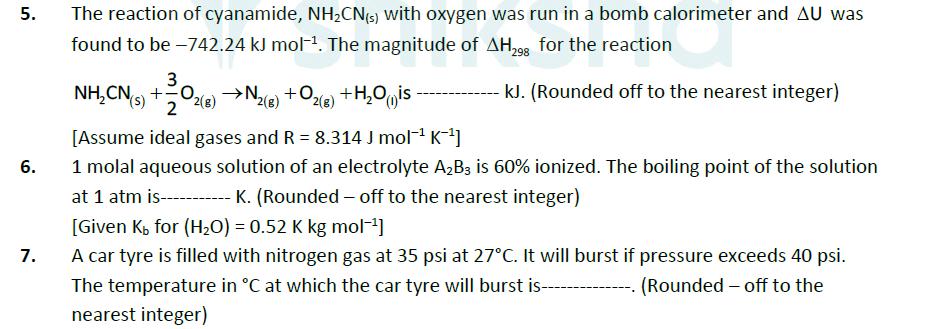
5. The reaction of cyanamide, NHCN(s) with oxygen was run in a bomb calorimeter and AU was found to be -742.24 kJ mol. The magnitude of AH298 for the reaction NH,CN, +0, 2(g) 2 N2(g) + O2(g) +H2O is - kJ. (Rounded off to the nearest integer) 6. 7. [Assume ideal gases and R = 8.314 J mol K-] 1 molal aqueous solution of an electrolyte A2B3 is 60% ionized. The boiling point of the solution K. (Rounded-off to the nearest integer) at 1 atm is [Given K for (H2O) = 0.52 K kg mol-] A car tyre is filled with nitrogen gas at 35 psi at 27C. It will burst if pressure exceeds 40 psi. The temperature in C at which the car tyre will burst is-- nearest integer) (Rounded-off to the
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


