Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8. H2S (5 moles) reacts completely with acidified aqueous potassium permanganate solution. In this reaction, the number of moles of water produced is x,

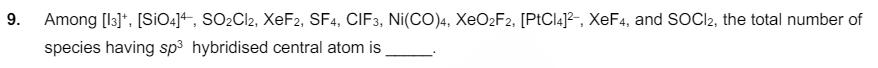
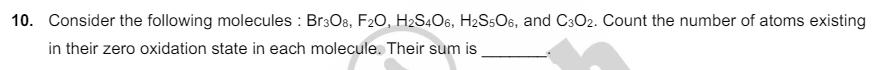

8. H2S (5 moles) reacts completely with acidified aqueous potassium permanganate solution. In this reaction, the number of moles of water produced is x, and the number of moles of electrons involved is y. The value of (x + y) is. 9. Among [13]. [SiO4], SO2Cl2, XeF2, SF4, CIF3, Ni(CO)4, XeO2F2, [PtCl4]2, XeF4, and SOCl2, the total number of species having sp hybridised central atom is 10. Consider the following molecules: Br308, F20, H2S406, H2S506, and C3O2. Count the number of atoms existing in their zero oxidation state in each molecule. Their sum is 11. For Het, a transition takes place from the orbit of radius 105.8 pm to the orbit of radius 26.45 pm. The wavelength (in nm) of the emitted photon during the transition is [Use: Bohr radius, a 52.9 pm Aaka BYJU'S Rydberg constant, RH = 2.2 10-18 J Planck's constant, h = 6.6 10-34 Js Speed of light, c = 3 108 ms 1]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


