Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The entropy versus temperature plot for phases a and at 1 bar pressure is given. ST and So are entropies of the phases at
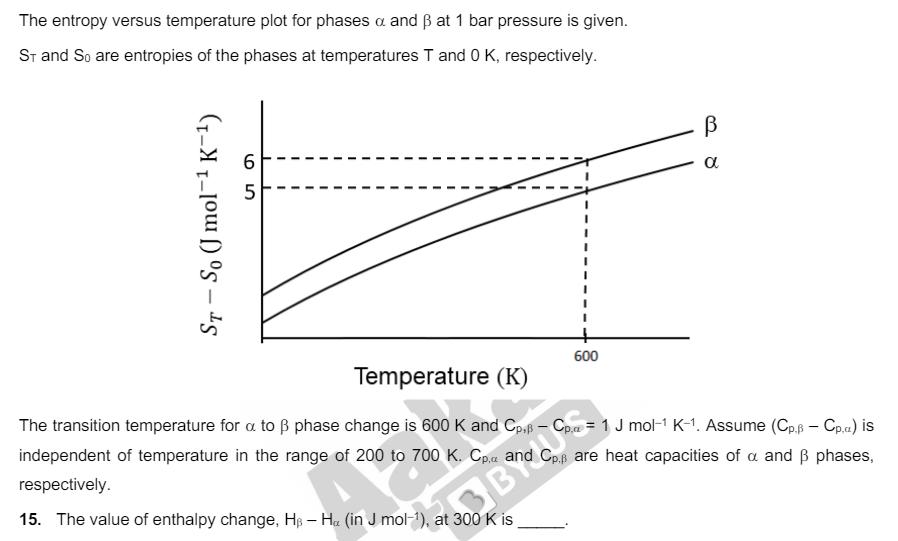

The entropy versus temperature plot for phases a and at 1 bar pressure is given. ST and So are entropies of the phases at temperatures T and 0 K, respectively. 65 ST - So (J mol K-) 600 R8 Temperature (K) The transition temperature for a to phase change is 600 K and Cp. - Cp. = 1 J mol-1 K-1. Assume (Cp. - Cp.a.) is independent of temperature in the range of 200 to 700 K. Cp, and Cp. are heat capacities of a and phases, respectively. 15. The value of enthalpy change, H - H (in J mol-1), at 300 K is. A trinitro compound, 1,3,5-tris-(4-nitrophenyl)benzene, on complete reaction with an excess of Sn/HCI gives a major product, which on treatment with an excess of NaNO2/HCI at 0C provides P as the product. P, upon treatment with excess of H2O at room temperature, gives the product Q. Bromination of Q in aqueous medium furnishes the product R. The compound P upon treatment with an excess of phenol under basic conditions gives the product S. The molar mass difference between compounds Q and R is 474 g mol-1 and between compounds P and S is 172.5 g mol-1. 16. The number of heteroatoms present in one molecule of R is [Use: Molar mass (in g mol-1): H = 1, C = 12, N = 14, O = 16, Br = 80, CI = 35.5 Atoms other than C and H are considered as heteroatoms]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


