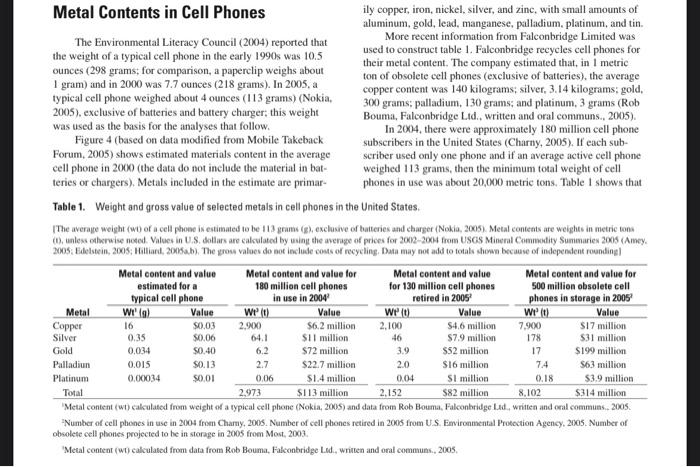
8. [Table 1 on page 2 (not the graph))] Use table 1 on page 2 to calculate the value of gold obtained for the total number of phones recycled above, THEN calculate the total value of gold in all phones in use using my '.com' estimate. For each calculation, show your work, and include your units. Metal Contents in Cell Phones ily copper, iron, nickel, silver, and zinc, with small amounts of aluminum, gold, lead, manganese, palladium, platinum, and tin. The Environmental Literacy Council (2004) reported that More recent information from Falconbridge Limited was the weight of a typical cell phone in the early 1990s was 10.5 used to construct table 1. Falconbridge recycles cell phones for ounces (298 grams; for comparison, a paperclip weighs about their metal content. The company estimated that, in I metric 1 gram) and in 2000 was 7.7 ounces ( 218 grams). In 2005, a ton of obsolete cell phones (exclusive of batteries), the average typical cell phone weighed about 4 ounces (113 grams) (Nokia _ copper content was 140 kilograms; silver, 3.14 kilograms; gold, 2005), exclusive of batteries and battery charger; this weight 300 grams; palladium, 130 grams; and platinum, 3 grams (Rob was used as the basis for the analyses that follow. Bouma, Falconbridge Lid., written and oral communs., 2005). Figure 4 (based on data modified from Mobile Takeback In 2004, there were approximately 180 million cell phone Forum, 2005) shows estimated materials content in the average scriber used only one phone and if an average active cell phone cell phone in 2000 (the data do not include the material in bat-_ weighed 113 grams, then the minimum total weight of cell teries or chargers). Metals included in the estimate are primar- phones in use was about 20,000 metric tons. Table 1 shows that Table 1. Weight and gross value of selected metals in cell phones in the United States. TThe average weight (w0) of a cell phoso is estinated to be 113 grami (g), exclusive of taiteries and charger (Nokia, 2005). Metal contents are weights in metric tans (1). unless ctherwise noed. Values in U.S. dotlars are calculated by using the averige of prices for 20022004 from USGS Mineral Commodity Suminaries 2005 (Amey. 'Metal content (wi) cakculated from weight of a typical cell phone (Nokia, 2005) and data from Rob Bouma, Falconbridge Ldd, written and oral commuas.. 2005. "Number of cell phones in use in 2004 from Chamy, 2005. Number of cell phones retired in 2005 from U. S. Emvironmental Protection Agency, 2005. Number of obsolete cell phohes projected to be in storage in 2005 from Most, 2003. 'Metal content (wi) calculated from data from Rob Bouma, Falconbridge L.d., writen and ofal communs., 2005. 8. [Table 1 on page 2 (not the graph))] Use table 1 on page 2 to calculate the value of gold obtained for the total number of phones recycled above, THEN calculate the total value of gold in all phones in use using my '.com' estimate. For each calculation, show your work, and include your units. Metal Contents in Cell Phones ily copper, iron, nickel, silver, and zinc, with small amounts of aluminum, gold, lead, manganese, palladium, platinum, and tin. The Environmental Literacy Council (2004) reported that More recent information from Falconbridge Limited was the weight of a typical cell phone in the early 1990s was 10.5 used to construct table 1. Falconbridge recycles cell phones for ounces (298 grams; for comparison, a paperclip weighs about their metal content. The company estimated that, in I metric 1 gram) and in 2000 was 7.7 ounces ( 218 grams). In 2005, a ton of obsolete cell phones (exclusive of batteries), the average typical cell phone weighed about 4 ounces (113 grams) (Nokia _ copper content was 140 kilograms; silver, 3.14 kilograms; gold, 2005), exclusive of batteries and battery charger; this weight 300 grams; palladium, 130 grams; and platinum, 3 grams (Rob was used as the basis for the analyses that follow. Bouma, Falconbridge Lid., written and oral communs., 2005). Figure 4 (based on data modified from Mobile Takeback In 2004, there were approximately 180 million cell phone Forum, 2005) shows estimated materials content in the average scriber used only one phone and if an average active cell phone cell phone in 2000 (the data do not include the material in bat-_ weighed 113 grams, then the minimum total weight of cell teries or chargers). Metals included in the estimate are primar- phones in use was about 20,000 metric tons. Table 1 shows that Table 1. Weight and gross value of selected metals in cell phones in the United States. TThe average weight (w0) of a cell phoso is estinated to be 113 grami (g), exclusive of taiteries and charger (Nokia, 2005). Metal contents are weights in metric tans (1). unless ctherwise noed. Values in U.S. dotlars are calculated by using the averige of prices for 20022004 from USGS Mineral Commodity Suminaries 2005 (Amey. 'Metal content (wi) cakculated from weight of a typical cell phone (Nokia, 2005) and data from Rob Bouma, Falconbridge Ldd, written and oral commuas.. 2005. "Number of cell phones in use in 2004 from Chamy, 2005. Number of cell phones retired in 2005 from U. S. Emvironmental Protection Agency, 2005. Number of obsolete cell phohes projected to be in storage in 2005 from Most, 2003. 'Metal content (wi) calculated from data from Rob Bouma, Falconbridge L.d., writen and ofal communs., 2005