Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-146 Liquid water enters an adiabatic piping system at 15C at a rate of 5 kg/s. It is observed that the water temperature rises
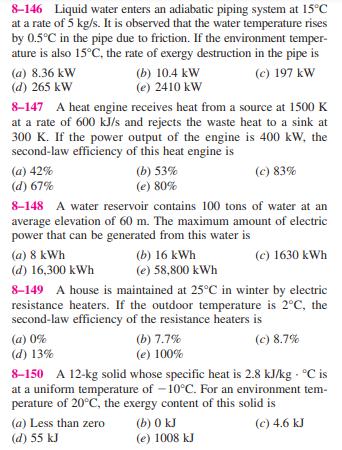
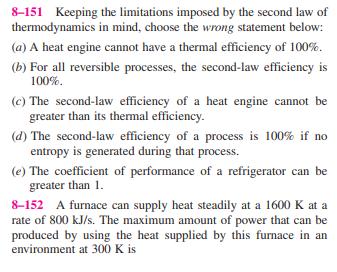
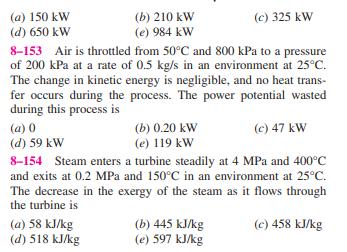
8-146 Liquid water enters an adiabatic piping system at 15C at a rate of 5 kg/s. It is observed that the water temperature rises by 0.5C in the pipe due to friction. If the environment temper- ature is also 15C, the rate of exergy destruction in the pipe is (b) 10.4 kW (a) 8.36 kW (d) 265 kW (e) 2410 kW (c) 197 kW 8-147 A heat engine receives heat from a source at 1500 K at a rate of 600 kJ/s and rejects the waste heat to a sink at 300 K. If the power output of the engine is 400 kW, the second-law efficiency of this heat engine is (a) 42% (d) 67% (b) 53% (e) 80% (c) 83% 8-148 A water reservoir contains 100 tons of water at an average elevation of 60 m. The maximum amount of electric power that can be generated from this water is (a) 8 kWh (d) 16,300 kWh (b) 16 kWh (e) 58,800 kWh (c) 1630 kWh 8-149 A house is maintained at 25C in winter by electric resistance heaters. If the outdoor temperature is 2C, the second-law efficiency of the resistance heaters is (a) 0% (d) 13% (b) 7.7% (e) 100% (c) 8.7% 8-150 A 12-kg solid whose specific heat is 2.8 kJ/kg C is at a uniform temperature of -10C. For an environment tem- perature of 20C, the exergy content of this solid is (a) Less than zero (d) 55 kJ (b) 0 kJ (e) 1008 kJ (c) 4.6 kJ 8-151 Keeping the limitations imposed by the second law of thermodynamics in mind, choose the wrong statement below: (a) A heat engine cannot have a thermal efficiency of 100%. (b) For all reversible processes, the second-law efficiency is 100%. (c) The second-law efficiency of a heat engine cannot be greater than its thermal efficiency. (d) The second-law efficiency of a process is 100% if no entropy is generated during that process. (e) The coefficient of performance of a refrigerator can be greater than 1. 8-152 A furnace can supply heat steadily at a 1600 K at a rate of 800 kJ/s. The maximum amount of power that can be produced by using the heat supplied by this furnace in an environment at 300 K is (a) 150 kW (d) 650 kW (b) 210 kW (e) 984 kW (c) 325 kW 8-153 Air is throttled from 50C and 800 kPa to a pressure of 200 kPa at a rate of 0.5 kg/s in an environment at 25C. The change in kinetic energy is negligible, and no heat trans- fer occurs during the process. The power potential wasted during this process is (a) 0 (d) 59 kW (b) 0.20 kW (e) 119 kW (c) 47 kW 8-154 Steam enters a turbine steadily at 4 MPa and 400C and exits at 0.2 MPa and 150C in an environment at 25C. The decrease in the exergy of the steam as it flows through the turbine is (a) 58 kJ/kg (d) 518 kJ/kg (b) 445 kJ/kg (e) 597 kJ/kg (c) 458 kJ/kg
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


