Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-31 The radiator of a steam heating system has a volume of 20 L and is filled with superheated water vapor at 200 kPa
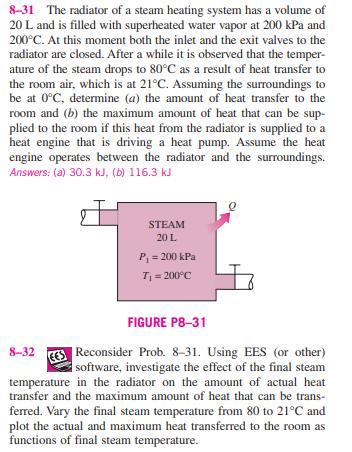


8-31 The radiator of a steam heating system has a volume of 20 L and is filled with superheated water vapor at 200 kPa and 200C. At this moment both the inlet and the exit valves to the radiator are closed. After a while it is observed that the temper- ature of the steam drops to 80C as a result of heat transfer to the room air, which is at 21C. Assuming the surroundings to be at 0C, determine (a) the amount of heat transfer to the room and (b) the maximum amount of heat that can be sup- plied to the room if this heat from the radiator is supplied to a heat engine that is driving a heat pump. Assume the heat engine operates between the radiator and the surroundings. Answers: (a) 30.3 kJ, (b) 116.3 kJ STEAM 20 L P = 200 kPa T =200C 8-32 FIGURE P8-31 Reconsider Prob. 8-31. Using EES (or other) software, investigate the effect of the final steam temperature in the radiator on the amount of actual heat transfer and the maximum amount of heat that can be trans- ferred. Vary the final steam temperature from 80 to 21C and plot the actual and maximum heat transferred to the room as functions of final steam temperature. 8-33E A well-insulated rigid tank contains 6 Ibm of satu- rated liquid-vapor mixture of water at 35 psia. Initially, three-quarters of the mass is in the liquid phase. An electric resistance heater placed in the tank is turned on and kept on until all the liquid in the tank is vaporized. Assuming the surroundings to be at 75F and 14.7 psia, determine (a) the exergy destruction and (b) the second-law efficiency for this process. 8-34 A rigid tank is divided into two equal parts by a parti- tion. One part of the tank contains 1.5 kg of compressed liq- uid water at 300 kPa and 60C and the other side is evacuated. Now the partition is removed, and the water expands to fill the entire tank. If the final pressure in the tank is 15 kPa, deter- mine the exergy destroyed during this process. Assume the surroundings to be at 25C and 100 kPa. Answer: 3.67 kJ 8-35 EES Reconsider Prob. 8-34. Using EES (or other) software, study the effect of final pressure in the tank on the exergy destroyed during the process. Plot the exergy destroyed as a function of the final pressure for final pressures between 25 and 15 kPa, and discuss the results. 8-31 The radiator of a steam heating system has a volume of 20 L and is filled with superheated water vapor at 200 kPa and 200C. At this moment both the inlet and the exit valves to the radiator are closed. After a while it is observed that the temper- ature of the steam drops to 80C as a result of heat transfer to the room air, which is at 21C. Assuming the surroundings to be at 0C, determine (a) the amount of heat transfer to the room and (b) the maximum amount of heat that can be sup- plied to the room if this heat from the radiator is supplied to a heat engine that is driving a heat pump. Assume the heat engine operates between the radiator and the surroundings. Answers: (a) 30.3 kJ, (b) 116.3 kJ STEAM 20 L P = 200 kPa T =200C 8-32 FIGURE P8-31 Reconsider Prob. 8-31. Using EES (or other) software, investigate the effect of the final steam temperature in the radiator on the amount of actual heat transfer and the maximum amount of heat that can be trans- ferred. Vary the final steam temperature from 80 to 21C and plot the actual and maximum heat transferred to the room as functions of final steam temperature. 8-33E A well-insulated rigid tank contains 6 Ibm of satu- rated liquid-vapor mixture of water at 35 psia. Initially, three-quarters of the mass is in the liquid phase. An electric resistance heater placed in the tank is turned on and kept on until all the liquid in the tank is vaporized. Assuming the surroundings to be at 75F and 14.7 psia, determine (a) the exergy destruction and (b) the second-law efficiency for this process. 8-34 A rigid tank is divided into two equal parts by a parti- tion. One part of the tank contains 1.5 kg of compressed liq- uid water at 300 kPa and 60C and the other side is evacuated. Now the partition is removed, and the water expands to fill the entire tank. If the final pressure in the tank is 15 kPa, deter- mine the exergy destroyed during this process. Assume the surroundings to be at 25C and 100 kPa. Answer: 3.67 kJ 8-35 EES Reconsider Prob. 8-34. Using EES (or other) software, study the effect of final pressure in the tank on the exergy destroyed during the process. Plot the exergy destroyed as a function of the final pressure for final pressures between 25 and 15 kPa, and discuss the results.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


