Answered step by step
Verified Expert Solution
Question
1 Approved Answer
89. Calculate the pH of a 10M solution of Ba(OH)2 if it undergoes complete ionisation. (K = 1 x 1014) - (1) 12.30 (3)
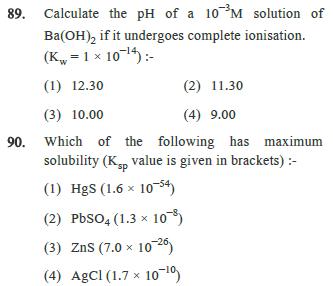

89. Calculate the pH of a 10M solution of Ba(OH)2 if it undergoes complete ionisation. (K = 1 x 1014) - (1) 12.30 (3) 10.00 (2) 11.30 (4) 9.00 90. Which of the following has maximum solubility (Ksp value is given in brackets) :- (1) HgS (1.6 x 10-54) (2) PbSO4 (1.3 x 10-) (3) ZnS (7.0 1026) (4) AgCl (1.7 x 10-10) 91. Two substance A(t1/2 = 5 min) and B(t1/2 = 15 min) follow first order kinetics are taken in such way that initially [A] = 4[B] then time after which the concentration of both the substance will be equal :- (1) 20 min (3) 50 min (2) 5 min (4) 15 min 92. Rate constant for a chemical reaction at 300 k is expressed as k A e 200, then activation energy of reaction is - (1) 200 kcal/mol (2) 120 cal/mol (3) 120 kcal/mol (4) 300 cal/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1
89 To calculate the pH of a 010 M solution of BaOH2 which undergoes complete ionization we need to consider that each mole of BaOH2 will produce 2 mol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


