Answered step by step
Verified Expert Solution
Question
1 Approved Answer
93. k=0.1 M min-1 Consider a reaction A(g) 2B(g). If initial concentration of A is 0.5 M, then select correct graph. (1) 0.5M (2)
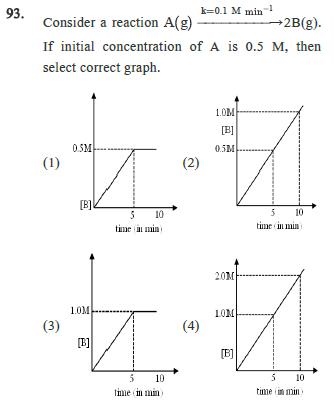
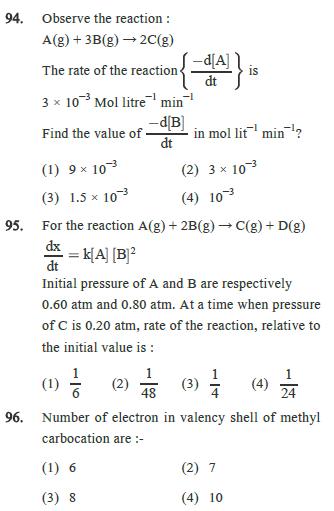
93. k=0.1 M min-1 Consider a reaction A(g) 2B(g). If initial concentration of A is 0.5 M, then select correct graph. (1) 0.5M (2) [B] time in min (3) 1.0M [B]] 1.0M [B]] 0.5M 5 10 5 10 time in min 20M LOM (4) 10 time in mind [B] 5 10 time in min 94. Observe the reaction: A(g) + 3B(g) 2C(g) The rate of the reaction 3 103 Mol litre min is dt -d[B] Find the value of in mol lit min? dt (1) 9 103 (3) 1.5 x 103 (2) 3 103 (4) 103 95. For the reaction A(g) + 2B(g) C(g) + D(g) dx dt = k[A] [B] Initial pressure of A and B are respectively 0.60 atm and 0.80 atm. At a time when pressure of C is 0.20 atm, rate of the reaction, relative to the initial value is : (1) (2) 48 (3)(4) 24 96. Number of electron in valency shell of methyl carbocation are :- (1) 6 (2) 7 (3) 8 (4) 10
Step by Step Solution
There are 3 Steps involved in it
Step: 1
93 The correct graph for the reaction Ag 2Bg with ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


