Question
8.Model 2 is the mass spectrum that resulted from the experiment in Model 1. a.What is the mass number of the most common isotope of
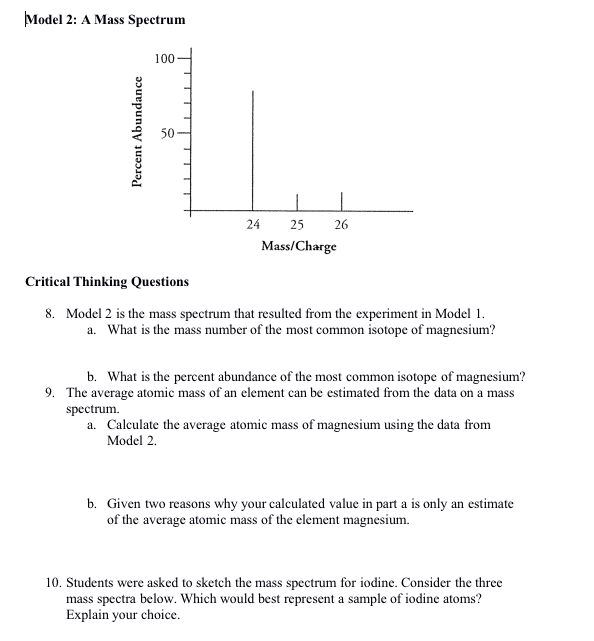
8.Model 2 is the mass spectrum that resulted from the experiment in Model 1. a.What is the mass number of the most common isotope of magnesium? b.What is the percent abundance of the most common isotope of magnesium?
9.The average atomic mass of an element can be estimated from the data on a mass spectrum. a.Calculate the average atomic mass of magnesium using the data from Model 2. b.Given two reasons why your calculated value in part a is only an estimate of the average atomic mass of the element magnesium.
10.Students were asked to sketch the mass spectrum for iodine. Consider the three mass spectra below. Which would best represent a sample of iodine atoms? Explain your choice.
11.The table below provides the mass number and percent abundance information for the element lead. Draw a mass spectrum for lead. (You can assume only +1 ions of lead are formed.) 204Pb 1.4% 206Pb 24.1% 207Pb 22.1% 208Pb 52.4%
Model 2: A Mass Spectrum 100 Percent Abundance 24 25 26 Mass/Charge Critical Thinking Questions 8. Model 2 is the mass spectrum that resulted from the experiment in Model 1. a. What is the mass number of the most common isotope of magnesium? b. What is the percent abundance of the most common isotope of magnesium? 9. The average atomic mass of an element can be estimated from the data on a mass spectrum. a. Calculate the average atomic mass of magnesium using the data from Model 2. b. Given two reasons why your calculated value in part a is only an estimate of the average atomic mass of the element magnesium. 10. Students were asked to sketch the mass spectrum for iodine. Consider the three mass spectra below. Which would best represent a sample of iodine atoms? Explain your choiceStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


