Answered step by step
Verified Expert Solution
Question
1 Approved Answer
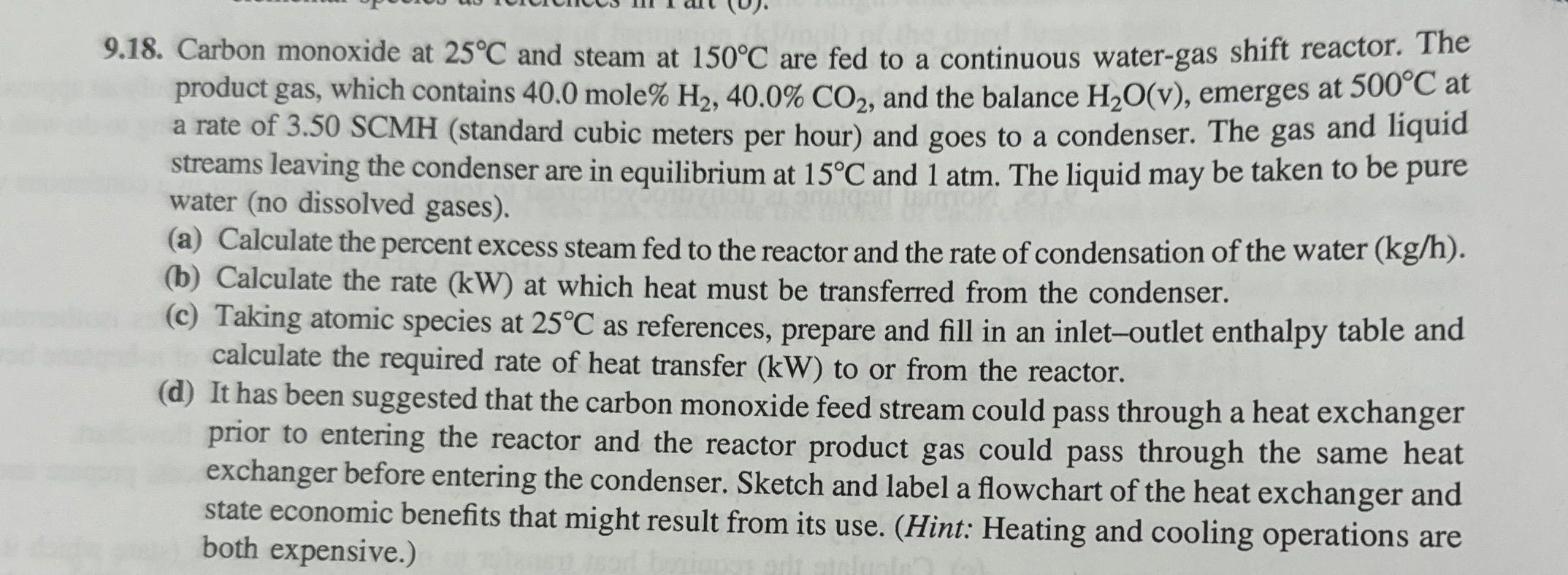
9 . 1 8 . Carbon monoxide at 2 5 C and steam at 1 5 0 C are fed to a continuous water -
Carbon monoxide at and steam at are fed to a continuous watergas shift reactor. The product gas, which contains mole and the balance emerges at at a rate of SCMH standard cubic meters per hour and goes to a condenser. The gas and liquid streams leaving the condenser are in equilibrium at and atm The liquid may be taken to be pure water no dissolved gases
a Calculate the percent excess steam fed to the reactor and the rate of condensation of the water
b Calculate the rate at which heat must be transferred from the condenser.
c Taking atomic species at as references, prepare and fill in an inletoutlet enthalpy table and calculate the required rate of heat transfer to or from the reactor.
d It has been suggested that the carbon monoxide feed stream could pass through a heat exchanger prior to entering the reactor and the reactor product gas could pass through the same heat exchanger before entering the condenser. Sketch and label a flowchart of the heat exchanger and state economic benefits that might result from its use. Hint: Heating and cooling operations are both expensive.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


