Question: 9, 10, 11, and 12 please show all work! thank you! II. Process Description: Boiler-Injection, Wet-Limestone Process The plant to be described is to produce




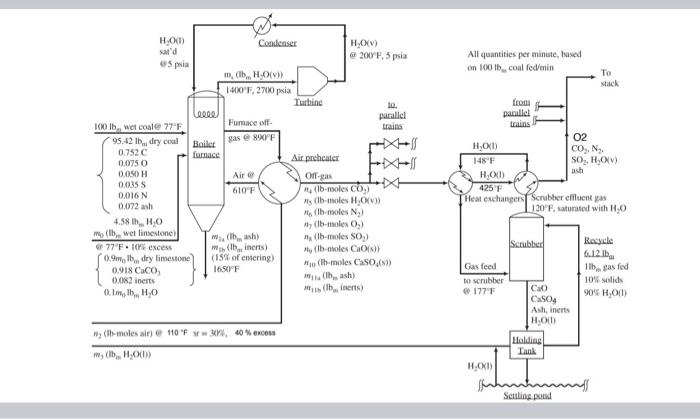
II. Process Description: Boiler-Injection, Wet-Limestone Process The plant to be described is to produce 500MW of electrical power. The flow rates, compositions, stream conditions, and other details to be given are representative of such installations. The key step in removing SO2 from the stack gas is the reaction of SO2 with CaO and oxygen to produce CaSO4, an insoluble stable compound. Four major components of the process will be traced: the coal-limestone-stack gas streams, the scrubber water, the cooling-heating water cycle, and the generated steam cycle. The composition of coal can vary considerably, but that shown in Table A is typical of that used in this process. During coal combustion the sulfur in the coal reacts to form SO2 and very small amounts of SO3. Eighty-five percent of the ash in the coal leaves the boiler in the stack gas as fly ash; nitrogen emerges as N2, and the carbon, hydrogen, and sulfur in the fuel are oxidized completely to CO2,H2O, and SO2. Mo1sture: 4.2810m/UU10m wet coal 2. Heat capacities Dry coal: CP=0.25 Btu/b m Ash: Cp=0.22Btu/bm+F 3. Heating value of coal: 13,240Btu/lbm dry coal Finely ground limestone, whose composition is given in Table B, is injected directly into the furnace where complete calcination occurs. CaCO3CaO+CO2 The limestone feed rate to the furnace is 10% in excess of that required for complete consumption of the generated SO2. Both limestone and coal enter the process at about 77F. A waste stream consisting of 15% of the limestone inerts and coal ash is removed from the furnace at 1650F.25% of the CaO produced from the limestone degradation reacts with SO2 in the furnace. Air at 110F and 30% relative humidity is brought to 610F in an air preheater, and the heated air is fed to the fumace. The air feed rate is 40% in excess of that required to bum the coal completely. Gases from the furnace containing fly ash, CaO, and CaSO4 and at 890F are cooled in the air preheater and then split into three trains. The gas in each train is cooled further to 177F, and fed to a serubber where it is contacted with an aqueous slurry of CaO and CaSO4. Sulfur dioxide is absorbed in the slurry and reacts with the CaO. The gas leaving each scrubber contains 3.333% of the SO2 and 0.3% of the fly ash emitted from the boiler fumace. The effluent gas from the scrubber, which is at 120F and saturated with water, is heated and mixed with the gas streams from the other trains. The combined gas stream is sent to a blower where its pressure is increased from 13.3 psia to 14.8 psia; it is then exhausted through a stack to the atmosphere. The liquid feed enters the serubber at 117F and contains 10.00 wt\% solids; it is fed at a rate such that there are 6.12lbbw liquid per tbw inlet gas. Liquid scrubber effluent at 120F is sent to a holding tank where it is mixed with fresh makeup water and water recycled from a settling pond. From the holding tank, one stream is recycled to serve as liquid feed to the scrubber and another is pumped to the settling pond for solids removal. Generation of steam and its utilization in the production of electricity in this plant is typical of many power cycles. Steam is generated in the boiler and leaves the boiler and superheater tubes at 1400F and 2700 psia. It is expanded through a turbine where its pressure and temperature are reduced to 5 psia and 200F. The low-pressure steam is then condensed at constant pressure and pumped isothermally to the inlet boiler nubes. The temperature of the water used to cool the gas entering the scrubber is 148F. The hot water at 425F is then used to reheat the effluent gas stream from the scrubber. (The water thus undergoes a closed cycle.) The power company for which you work is contemplating adding an SO2 scrubber to one of its generation stations and has asked you to do the preliminary process evaluation. In solving the following problems, you may neglect the formation of SO in the furnace, and assume that CaSO4 and CaO are the only calcium compounds present in the slurry used in the scrubber (i.e., neglect the sulfite, bisulfite, and bisulfate compounds that are present to some extent in the real process). III. Problems Take 100lb/m/min of wet coal fed to the boiler as the basis for the calculation of the process. CS 1. (20 pts) Construct a flowchart of the process, labeling all process streams. Show the details of only one train in the SO2 scrubber operation. CS 2. (10 pts) From the data on coal composition given in Table , determine the molar flow rate of each element other than ash in the dry coal. CS 3. (10 pts) Determine the feed rate of O2 required for complete combustion. CS 4. (10 pts) If 40% excess O2 is fed to the boiler, calculate: a. The air feed in i.lb-molemin.i.ii.Standardcubicfeetmin.Actualcubicfeet/min. b. The molar flow rate of water in the air stream CS 5. (10 pts) Determine the rate of flow of CaCO3, inerts, and H2O in the limestone feed. CS 6. (30 pts) Estimate the rate at which each component in the gas leaves the furnace. What is the waste removal rate from the boiler? CS 7. (30 pts) At what rate must heat be removed from the furtace? CS 8. (30 pts) Plants of the type under consideration operate at an efficiency of about 35%; that is, for each unit of heat extracted from the combustion process, 0.35 units are converted to electrical energy. From this efficiency and the specified power output of 500MW, determine: a. Coal feed rate in lbm/h. b. Air feed rate in i. lb-mole/min. ii. Standard cubic feet/min. c. The flow rate of each component in the gas leaving the furnace. CS 9. (20 pts) How much additional coal is consumed in the boiler because of the addition of limestone? CS 10. (10 pts) Calculate the feed rate of liquid to each scrubber in lbm/h. CS 11. (50 pts) Estimate the composition and flow rates of the gas and liquid streams leaving the scrubber. Are the EPA requirements satisfied? CS 12. (10 pts) Determine the rate at which water (fresh water and recycled water from the pond) must be mixed with the effluent from the scrubber to reduce the solids content (ash, CaO, CaSO4 ) to 10wt%. CS 13. (10 pts) If essentially all the solids in the waste stream fed to the settling pond are precipitated, and if the pond surface area is such that half of the water in the waste stream is evaporated, at what rate, in gallons per minute, must fresh water be fed to the process? CS 14. (30 pts) Determine the temperature of the gas stream as it leaves the heat exchanger following the boiler. CS 15. ( 30pts) What is the water circulation rate through the heat-recovery loop; that is, the flow rate of the stream that cools the gases entering the scrubber and heats the absorber effluent? What is the minimum pressure at which this cycle can operate with liquid water? To what temperature is the gas leaving the scrubber reheated before it is mixed with gas from other trains? CS 16. (30 pts) One of the design specifications for power plant boilers is the amount of excess air used in buming coal. Evaluate the heat removed from the boiler for 20% and 100% excess air if the temperature of the exit gases and slag is 890F. What are the ramifications of altering the ratio of air to coal? CS 17. (10 pts) At first glance it might appear that there is no need to split the exit gases into three streams, only to remix them later in the process. However, for the scrubbers under consideration, the maximum allowable velocity of the gas through the empty column is given by vm(ft/s)=0.15[(LG)G]0.5 where G and L are the densities of the gas and liquid phases. Estimate the minimum column diameters for one-, two-, and three-train operations. Why is the three-train operation used? II. Process Description: Boiler-Injection, Wet-Limestone Process The plant to be described is to produce 500MW of electrical power. The flow rates, compositions, stream conditions, and other details to be given are representative of such installations. The key step in removing SO2 from the stack gas is the reaction of SO2 with CaO and oxygen to produce CaSO4, an insoluble stable compound. Four major components of the process will be traced: the coal-limestone-stack gas streams, the scrubber water, the cooling-heating water cycle, and the generated steam cycle. The composition of coal can vary considerably, but that shown in Table A is typical of that used in this process. During coal combustion the sulfur in the coal reacts to form SO2 and very small amounts of SO3. Eighty-five percent of the ash in the coal leaves the boiler in the stack gas as fly ash; nitrogen emerges as N2, and the carbon, hydrogen, and sulfur in the fuel are oxidized completely to CO2,H2O, and SO2. Mo1sture: 4.2810m/UU10m wet coal 2. Heat capacities Dry coal: CP=0.25 Btu/b m Ash: Cp=0.22Btu/bm+F 3. Heating value of coal: 13,240Btu/lbm dry coal Finely ground limestone, whose composition is given in Table B, is injected directly into the furnace where complete calcination occurs. CaCO3CaO+CO2 The limestone feed rate to the furnace is 10% in excess of that required for complete consumption of the generated SO2. Both limestone and coal enter the process at about 77F. A waste stream consisting of 15% of the limestone inerts and coal ash is removed from the furnace at 1650F.25% of the CaO produced from the limestone degradation reacts with SO2 in the furnace. Air at 110F and 30% relative humidity is brought to 610F in an air preheater, and the heated air is fed to the fumace. The air feed rate is 40% in excess of that required to bum the coal completely. Gases from the furnace containing fly ash, CaO, and CaSO4 and at 890F are cooled in the air preheater and then split into three trains. The gas in each train is cooled further to 177F, and fed to a serubber where it is contacted with an aqueous slurry of CaO and CaSO4. Sulfur dioxide is absorbed in the slurry and reacts with the CaO. The gas leaving each scrubber contains 3.333% of the SO2 and 0.3% of the fly ash emitted from the boiler fumace. The effluent gas from the scrubber, which is at 120F and saturated with water, is heated and mixed with the gas streams from the other trains. The combined gas stream is sent to a blower where its pressure is increased from 13.3 psia to 14.8 psia; it is then exhausted through a stack to the atmosphere. The liquid feed enters the serubber at 117F and contains 10.00 wt\% solids; it is fed at a rate such that there are 6.12lbbw liquid per tbw inlet gas. Liquid scrubber effluent at 120F is sent to a holding tank where it is mixed with fresh makeup water and water recycled from a settling pond. From the holding tank, one stream is recycled to serve as liquid feed to the scrubber and another is pumped to the settling pond for solids removal. Generation of steam and its utilization in the production of electricity in this plant is typical of many power cycles. Steam is generated in the boiler and leaves the boiler and superheater tubes at 1400F and 2700 psia. It is expanded through a turbine where its pressure and temperature are reduced to 5 psia and 200F. The low-pressure steam is then condensed at constant pressure and pumped isothermally to the inlet boiler nubes. The temperature of the water used to cool the gas entering the scrubber is 148F. The hot water at 425F is then used to reheat the effluent gas stream from the scrubber. (The water thus undergoes a closed cycle.) The power company for which you work is contemplating adding an SO2 scrubber to one of its generation stations and has asked you to do the preliminary process evaluation. In solving the following problems, you may neglect the formation of SO in the furnace, and assume that CaSO4 and CaO are the only calcium compounds present in the slurry used in the scrubber (i.e., neglect the sulfite, bisulfite, and bisulfate compounds that are present to some extent in the real process). III. Problems Take 100lb/m/min of wet coal fed to the boiler as the basis for the calculation of the process. CS 1. (20 pts) Construct a flowchart of the process, labeling all process streams. Show the details of only one train in the SO2 scrubber operation. CS 2. (10 pts) From the data on coal composition given in Table , determine the molar flow rate of each element other than ash in the dry coal. CS 3. (10 pts) Determine the feed rate of O2 required for complete combustion. CS 4. (10 pts) If 40% excess O2 is fed to the boiler, calculate: a. The air feed in i.lb-molemin.i.ii.Standardcubicfeetmin.Actualcubicfeet/min. b. The molar flow rate of water in the air stream CS 5. (10 pts) Determine the rate of flow of CaCO3, inerts, and H2O in the limestone feed. CS 6. (30 pts) Estimate the rate at which each component in the gas leaves the furnace. What is the waste removal rate from the boiler? CS 7. (30 pts) At what rate must heat be removed from the furtace? CS 8. (30 pts) Plants of the type under consideration operate at an efficiency of about 35%; that is, for each unit of heat extracted from the combustion process, 0.35 units are converted to electrical energy. From this efficiency and the specified power output of 500MW, determine: a. Coal feed rate in lbm/h. b. Air feed rate in i. lb-mole/min. ii. Standard cubic feet/min. c. The flow rate of each component in the gas leaving the furnace. CS 9. (20 pts) How much additional coal is consumed in the boiler because of the addition of limestone? CS 10. (10 pts) Calculate the feed rate of liquid to each scrubber in lbm/h. CS 11. (50 pts) Estimate the composition and flow rates of the gas and liquid streams leaving the scrubber. Are the EPA requirements satisfied? CS 12. (10 pts) Determine the rate at which water (fresh water and recycled water from the pond) must be mixed with the effluent from the scrubber to reduce the solids content (ash, CaO, CaSO4 ) to 10wt%. CS 13. (10 pts) If essentially all the solids in the waste stream fed to the settling pond are precipitated, and if the pond surface area is such that half of the water in the waste stream is evaporated, at what rate, in gallons per minute, must fresh water be fed to the process? CS 14. (30 pts) Determine the temperature of the gas stream as it leaves the heat exchanger following the boiler. CS 15. ( 30pts) What is the water circulation rate through the heat-recovery loop; that is, the flow rate of the stream that cools the gases entering the scrubber and heats the absorber effluent? What is the minimum pressure at which this cycle can operate with liquid water? To what temperature is the gas leaving the scrubber reheated before it is mixed with gas from other trains? CS 16. (30 pts) One of the design specifications for power plant boilers is the amount of excess air used in buming coal. Evaluate the heat removed from the boiler for 20% and 100% excess air if the temperature of the exit gases and slag is 890F. What are the ramifications of altering the ratio of air to coal? CS 17. (10 pts) At first glance it might appear that there is no need to split the exit gases into three streams, only to remix them later in the process. However, for the scrubbers under consideration, the maximum allowable velocity of the gas through the empty column is given by vm(ft/s)=0.15[(LG)G]0.5 where G and L are the densities of the gas and liquid phases. Estimate the minimum column diameters for one-, two-, and three-train operations. Why is the three-train operation used
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


