Answered step by step
Verified Expert Solution
Question
1 Approved Answer
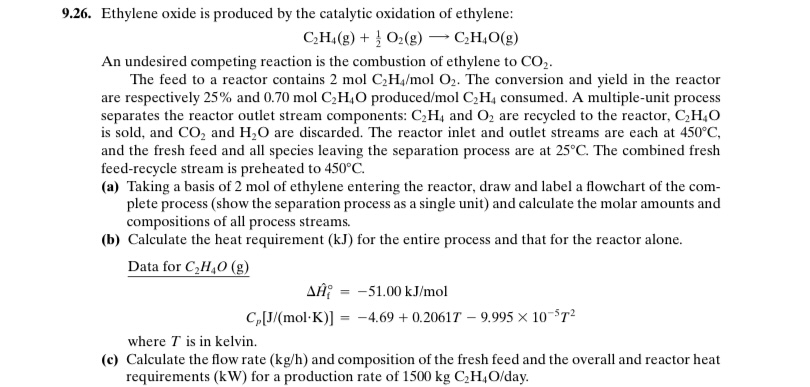
9 . 2 6 . Ethylene oxide is produced by the catalytic oxidation of ethylene: C 2 H 4 ( g ) + 1 2
Ethylene oxide is produced by the catalytic oxidation of ethylene:
An undesired competing reaction is the combustion of ethylene to
The feed to a reactor contains The conversion and yield in the reactor
are respectively and producedmol consumed. A multipleunit process
separates the reactor outlet stream components: and are recycled to the reactor,
is sold, and and are discarded. The reactor inlet and outlet streams are each at
and the fresh feed and all species leaving the separation process are at The combined fresh
feedrecycle stream is preheated to
a Taking a basis of mol of ethylene entering the reactor, draw and label a flowchart of the com
plete process show the separation process as a single unit and calculate the molar amounts and
compositions of all process streams.
b Calculate the heat requirement for the entire process and that for the reactor alone.
Data for
where is in kelvin.
c Calculate the flow rate and composition of the fresh feed and the overall and reactor heat
requirements for a production rate of day.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


