Answered step by step
Verified Expert Solution
Question
1 Approved Answer
9) Four litres of 0.4M 3-(N-morpholino) propane sulfonic acid (MOPS) buffer of pH 7.5 are required. Available in the laboratory are 10M HCI and
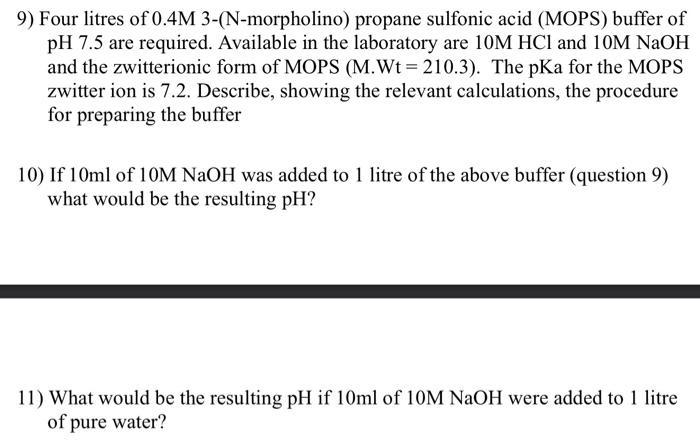
9) Four litres of 0.4M 3-(N-morpholino) propane sulfonic acid (MOPS) buffer of pH 7.5 are required. Available in the laboratory are 10M HCI and 10M NaOH and the zwitterionic form of MOPS (M.Wt=210.3). The pKa for the MOPS zwitter ion is 7.2. Describe, showing the relevant calculations, the procedure for preparing the buffer 10) If 10ml of 10M NaOH was added to 1 litre of the above buffer (question 9) what would be the resulting pH? 11) What would be the resulting pH if 10ml of 10M NaOH were added to 1 litre of pure water?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


