Answered step by step
Verified Expert Solution
Question
1 Approved Answer
9. Sucrose or common sugar (C12H22O11) is hydrolyzed with water to form fructose (C6H12O6) and glucose (C6H12O6). The mix of fructose and glucose is known
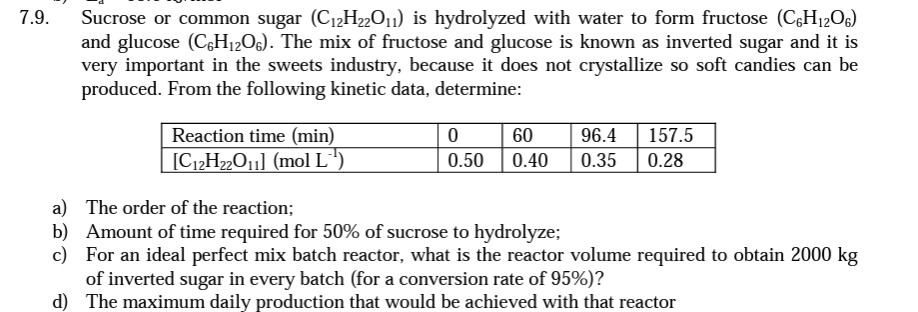 9. Sucrose or common sugar (C12H22O11) is hydrolyzed with water to form fructose (C6H12O6) and glucose (C6H12O6). The mix of fructose and glucose is known as inverted sugar and it is very important in the sweets industry, because it does not crystallize so soft candies can be produced. From the following kinetic data, determine: a) The order of the reaction; b) Amount of time required for 50% of sucrose to hydrolyze; c) For an ideal perfect mix batch reactor, what is the reactor volume required to obtain 2000kg of inverted sugar in every batch (for a conversion rate of 95% )? d) The maximum daily production that would be achieved with that reactor
9. Sucrose or common sugar (C12H22O11) is hydrolyzed with water to form fructose (C6H12O6) and glucose (C6H12O6). The mix of fructose and glucose is known as inverted sugar and it is very important in the sweets industry, because it does not crystallize so soft candies can be produced. From the following kinetic data, determine: a) The order of the reaction; b) Amount of time required for 50% of sucrose to hydrolyze; c) For an ideal perfect mix batch reactor, what is the reactor volume required to obtain 2000kg of inverted sugar in every batch (for a conversion rate of 95% )? d) The maximum daily production that would be achieved with that reactor Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


