9. The designers of a portable barbecue need to estimate the viscosity of a Propane/Isobutane gas mixture at atmospheric pressure. The mixture is 72%

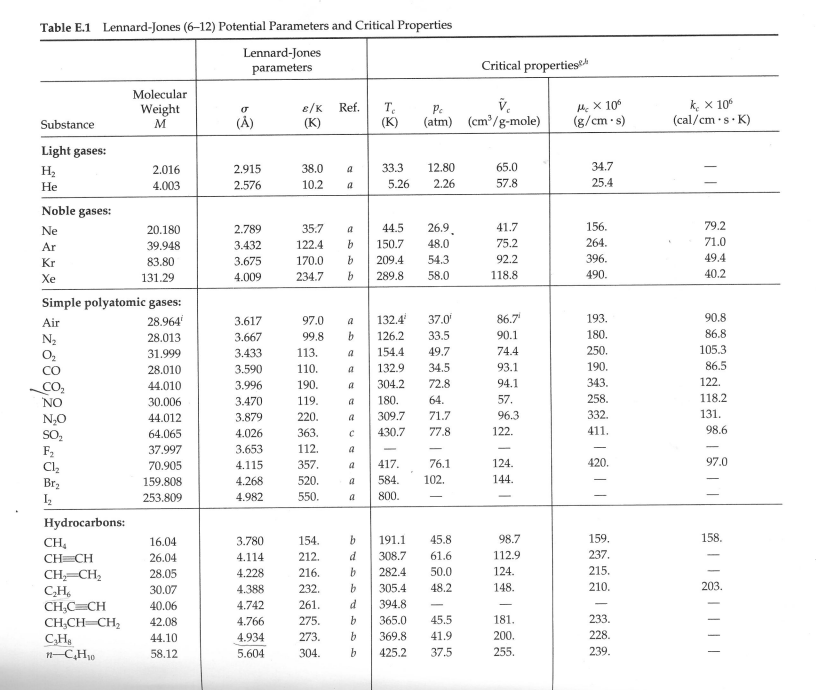
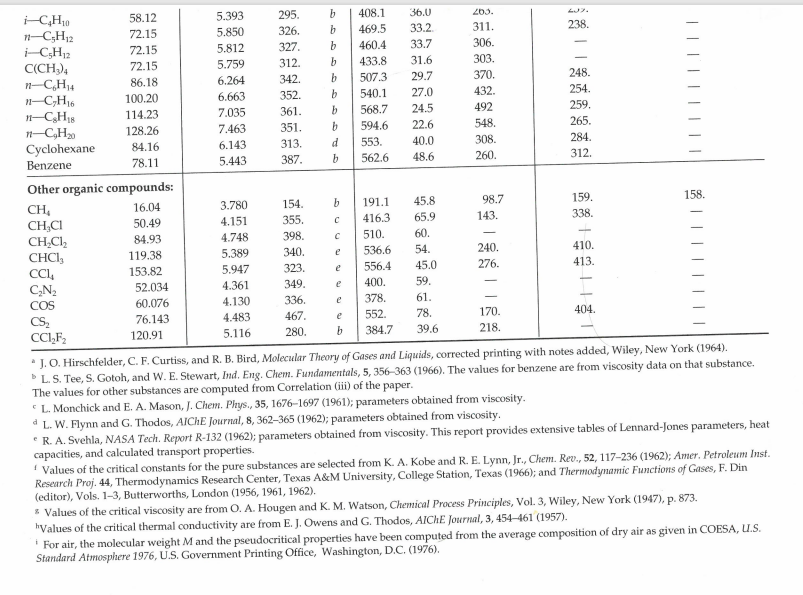
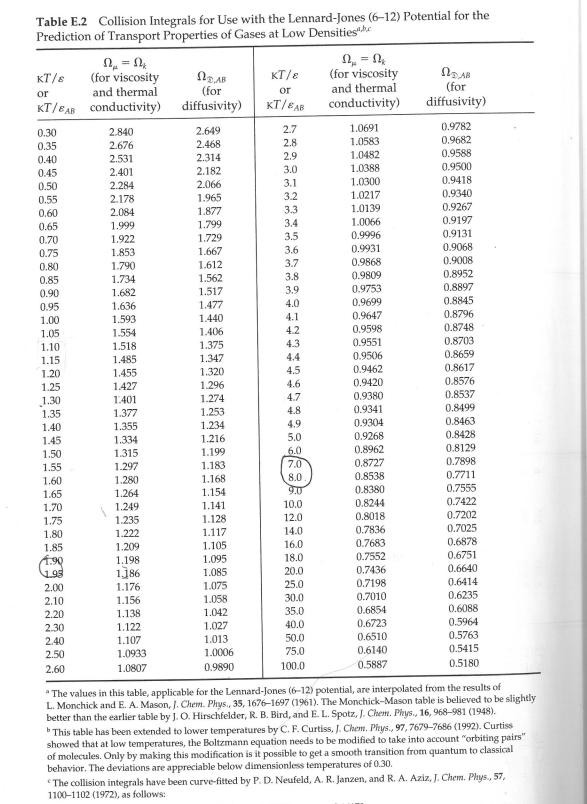
9. The designers of a portable barbecue need to estimate the viscosity of a Propane/Isobutane gas mixture at atmospheric pressure. The mixture is 72% (by mole fraction) Propane and the remainder is Butane. The mixture is at a temperature of 30 C. Under the prevailing conditions, the viscosity of butane is estimated at 7.53 x 10-6 Pas. Determine the viscosity of the Propane/Butane gas mixture. (Note - You must use the Chapman-Enskog equation to determine the viscosity of Propane, and then calculate the viscosity of the mixture using the mixture equation with interaction parameters). Table E.1 Lennard-Jones (6-12) Potential Parameters and Critical Properties Lennard-Jones parameters Critical properties Substance Molecular Weight M E/K () (K) Ref. T (K) Pc V (atm) (cm/g-mole) MX 106 (g/cms) k, x 10% (cal/cm.s.K) Light gases: H 2.016 2.915 38.0 a 33.3 12.80 65.0 34.7 He 4.003 2.576 10.2 a 5.26 2.26 57.8 25.4 Noble gases: Ne 20.180 2.789 35.7 a 44.5 26.9 41.7 156. 79.2 Ar 39.948 3.432 122.4 b 150.7 48.0 75.2 264. 71.0 Kr 83.80 3.675 170.0 b 209.4 54.3 92.2 396. 49.4 Xe 131.29 4.009 234.7 b 289.8 58.0 118.8 490. 40.2 Simple polyatomic gases: Air 28.964 3.617 97.0 a 132.4 37.0 86.7' 193. 90.8 N 28.013 3.667 99.8 b 126.2 33.5 90.1 180. 86.8 02 31.999 3.433 113. a 154.4 49.7 74.4 250. 105.3 28.010 3.590 110. a 132.9 34.5 93.1 190. 86.5 CO 44.010 3.996 190. a 304.2 72.8 94.1 343. 122. NO 30.006 3.470 119. a 180. 64. 57. 258. 118.2 NO 44.012 3.879 220. a 309.7 71.7 96.3 332. 131. SO 64.065 4.026 363. C 430.7 77.8 122. 411. 98.6 F 37.997 3.653 112. a - - - - Cl 70.905 4.115 357. a 417. 76.1 124. 420. 97.0 Br 159.808 4.268 520. a 584. 102. 144. I, 253.809 4.982 550. a 800. - Hydrocarbons: CH4 16.04 3.780 154. b 191.1 45.8 98.7 159. 158. CH=CH 26.04 4.114 212. d 308.7 61.6 112.9 237. CH2=CH2 28.05 4.228 216. b 282.4 50.0 124. 215. C2H6 30.07 4.388 232. b 305.4 48.2 148. 210. 203. CHC=CH 40.06 4.742 261. d 394.8 - CH3CH=CH2 42.08 4.766 275. b 365.0 45.5 181. 233. CHB 44.10 4.934 273. b 369.8 41.9 200. 228. n-CH 58.12 5.604 304. b 425.2 37.5 255. 239. i-C4H10 58.12 5.393 295. b 408.1 36.0 263. n1-C5H12 72.15 5.850 326. b 469.5 33.2. 311. 238. i-C5H12 72.15 5.812 327. b 460.4 33.7 306. - C(CH3)4 72.15 5.759 312. b 433.8 31.6 303. n-C6H14 86.18 6.264 342. b 507.3 29.7 370. 248. n-C,H16 100.20 6.663 352. b 540.1 27.0 432. 254. n-C8H18 114.23 7.035 361. b 568.7 24.5 492 259. n-C,H20 128.26 7.463 351. b 594.6 22.6 548. 265. Cyclohexane 84.16 6.143 313. d 553. 40.0 308. 284. Benzene 78.11 5.443 387. b 562.6 48.6 260. 312. Other organic compounds: CH 16.04 3.780 154. b 191.1 45.8 98.7 159. 158. CHCI 50.49 4.151 355. 416.3 65.9 143. 338. CHCl 84.93 4.748 398. C 510. 60. - CHCI, 119.38 5.389 340. e 536.6 54. 240. 410. CCl4 153.82 5.947 323. e 556.4 45.0 276. 413. CN 52.034 4.361 349. e 400. 59. 60.076 4.130 336. e 378. 61. CS 76.143 4.483 467. e 552. 78. 170. 404. CCF 120.91 5.116 280. b 384.7 39.6 218. *J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, corrected printing with notes added, Wiley, New York (1964). L. S. Tee, S. Gotoh, and W. E. Stewart, Ind. Eng. Chem. Fundamentals, 5, 356-363 (1966). The values for benzene are from viscosity data on that substance. The values for other substances are computed from Correlation (iii) of the paper. L. Monchick and E. A. Mason, J. Chem. Phys., 35, 1676-1697 (1961); parameters obtained from viscosity. dL. W. Flynn and G. Thodos, AIChE Journal, 8, 362-365 (1962); parameters obtained from viscosity. R. A. Svehla, NASA Tech. Report R-132 (1962); parameters obtained from viscosity. This report provides extensive tables of Lennard-Jones parameters, heat capacities, and calculated transport properties. Values of the critical constants for the pure substances are selected from K. A. Kobe and R. E. Lynn, Jr., Chem. Rev., 52, 117-236 (1962); Amer. Petroleum Inst. Research Proj. 44, Thermodynamics Research Center, Texas A&M University, College Station, Texas (1966); and Thermodynamic Functions of Gases, F. Din (editor), Vols. 1-3, Butterworths, London (1956, 1961, 1962). Values of the critical viscosity are from O. A. Hougen and K. M. Watson, Chemical Process Principles, Vol. 3, Wiley, New York (1947), p. 873. "Values of the critical thermal conductivity are from E. J. Owens and G. Thodos, AIChE Journal, 3, 454-461 (1957). For air, the molecular weight M and the pseudocritical properties have been computed from the average composition of dry air as given in COESA, U.S. Standard Atmosphere 1976, U.S. Government Printing Office, Washington, D.C. (1976). Table E.2 Collision Integrals for Use with the Lennard-Jones (6-12) Potential for the Prediction of Transport Properties of Gases at Low Densities" KT/B or =k (for viscosity and thermal KT/8 ,, = 2; (for viscosity (for KT/BAB conductivity) diffusivity) KT/BAB and thermal conductivity) (for diffusivity) 0.30 2.840 2.649 2.7 1.0691 0.9782 0.35 2.676 2.468 2.8 1.0583 0.9682 0.40 2.531 2.314 2.9 1.0482 0.9588 0.45 2.401 2.182 3.0 1.0388 0.9500 0.50 2.284 2.066 3.1 1.0300 0.9418 0.55 2.178 1.965 3.2 1.0217 0.9340 0.60 2.084 1.877 3.3 1.0139 0.9267 0.65 1.999 1.799 3.4 1.0066 0.9197 0.70 1.922 1.729 3.5 0.9996 0.9131 0.75 1.853 1.667 3.6 0.9931 0.9068 0.80 1.790 1.612 3.7 0.9868 0.9008 0.85 1.734 1.562 3.8 0.9809 0.8952 0.90 1.682 1.517 3.9 0.9753 0.8897 0.95 1.636 1.477 4.0 0.9699 0.8845 1.00 1.593 1.440 4.1 0.9647 0.8796 1.05 1.554 1.406 4.2 0.9598 0.8748 1.10 1.518 1.375 4.3 0.9551 0.8703 1.15 1.485 1.347 4.4 0.9506 0.8659 1.20 1.455 1.320 4.5 0.9462 0.8617 1.25 1.427 1.296 4.6 0.9420 0.8576 1.30 1.401 1.274 4.7 0.9380 0.8537 1.35 1.377 1.253 4.8 0.9341 0.8499 1.40 1.355 1.234 4.9 0.9304 0.8463 1.45 1.334 1.216 5.0 0.9268 0.8428 1.50 1.315 1.199 6.0 0.8962 0.8129 1.55 1.297 1.183 7.0 0.8727 0.7898 1.60 1.280 1.168 8.0 0.8538 0.7711 1.65 1.264 1.154 9.0 0.8380 0.7555 1.70 1.249 1.141 10.0 0.8244 0.7422 1.75 1.235 1.128 12.0 0.8018 0.7202 1.80 1.222 1.117 14.0 0.7836 0.7025 1.85 1.209 1.105 16.0 0.7683 0.6878 1.90 1.198 1.095 18.0 0.7552 0.6751 1.99 1,186 1.085 20.0 0.7436 0.6640 2.00 1.176 1.075 25.0 0.7198 0.6414 2.10 1.156 1.058 30.0 0.7010 0.6235 2.20 1.138 1.042 35.0 0.6854 0.6088 2.30 1.122 1.027 40.0 0.6723 0.5964 2.40 1.107 1.013 50.0 0.6510 0.5763 2.50 1.0933 1.0006 75.0 0.6140 0.5415 2.60 1.0807 0.9890 100.0 0.5887 0.5180 "The values in this table, applicable for the Lennard-Jones (6-12) potential, are interpolated from the results of L. Monchick and E. A. Mason, J. Chem. Phys., 35, 1676-1697 (1961). The Monchick-Mason table is believed to be slightly better than the earlier table by J. O. Hirschfelder, R. B. Bird, and E. L. Spotz, J. Chem. Phys., 16, 968-981 (1948). This table has been extended to lower temperatures by C. F. Curtiss, J. Chem. Phys., 97, 7679-7686 (1992). Curtiss showed that at low temperatures, the Boltzmann equation needs to be modified to take into account "orbiting pairs" of molecules. Only by making this modification is it possible to get a smooth transition from quantum to classical behavior. The deviations are appreciable below dimensionless temperatures of 0.30. "The collision integrals have been curve-fitted by P. D. Neufeld, A. R. Janzen, and R. A. Aziz, J. Chem. Phys., 57, 1100-1102 (1972), as follows:
Step by Step Solution
There are 3 Steps involved in it
Step: 1
the viscosit...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


