Answered step by step
Verified Expert Solution
Question
1 Approved Answer
9) What is the side arm of a filter flask for? 14) Once you have decided upon the solvent to use you can begin the
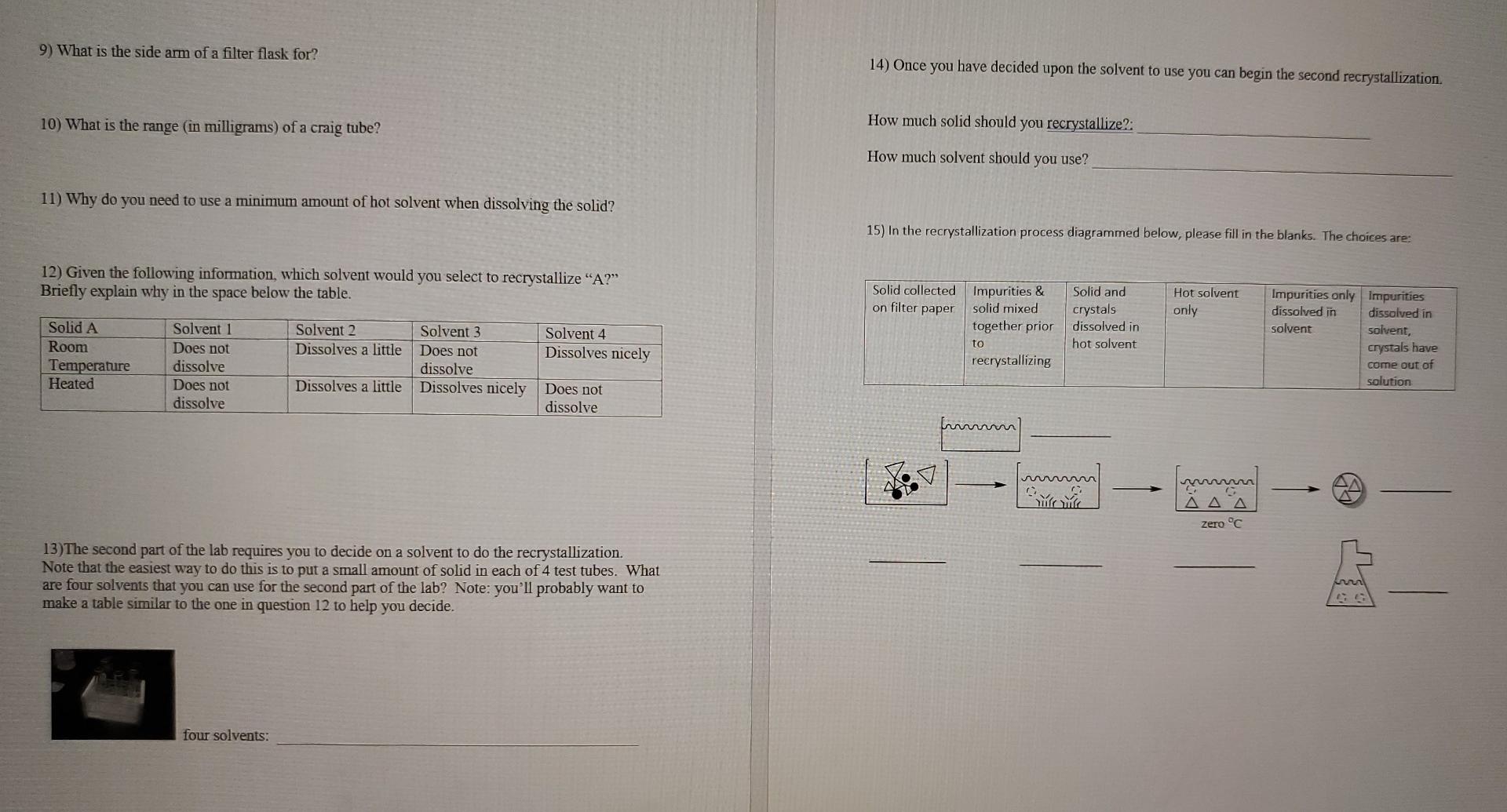
9) What is the side arm of a filter flask for? 14) Once you have decided upon the solvent to use you can begin the second recrystallization. 10) What is the range (in milligrams) of a craig tube? How much solid should you recrystallize?: How much solvent should you use? 11) Why do you need to use a minimum amount of hot solvent when dissolving the solid? 15) In the recrystallization process diagrammed below, please fill in the blanks. The choices are: 12) Given the following information, which solvent would you select to recrystallize "A?" Briefly explain why in the space below the table. Solid and crystals dissolved in hot solvent Hot solvent only Solid A Room Temperature Heated Solvent 2 Dissolves a little Solvent 1 Does not dissolve Does not dissolve Solvent 3 Does not dissolve Dissolves nicely Solvent 4 Dissolves nicely Solid collected Impurities & on filter paper solid mixed together prior to recrystallizing Impurities only impurities dissolved in dissolved in solvent solvent, crystals have come out of solution Dissolves a little Does not dissolve mm 1 ||) Yule Zero C 13)The second part of the lab requires you to decide on a solvent to do the recrystallization. Note that the easiest way to do this is to put a small amount of solid in each of 4 test tubes. What are four solvents that you can use for the second part of the lab? Note: you'll probably want to make a table similar to the one in question 12 to help you decide. four solvents
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


