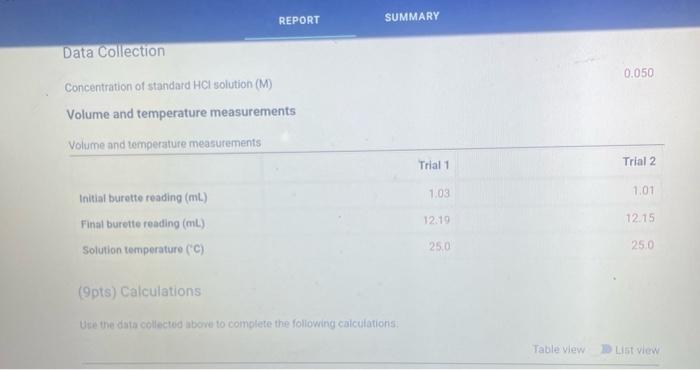
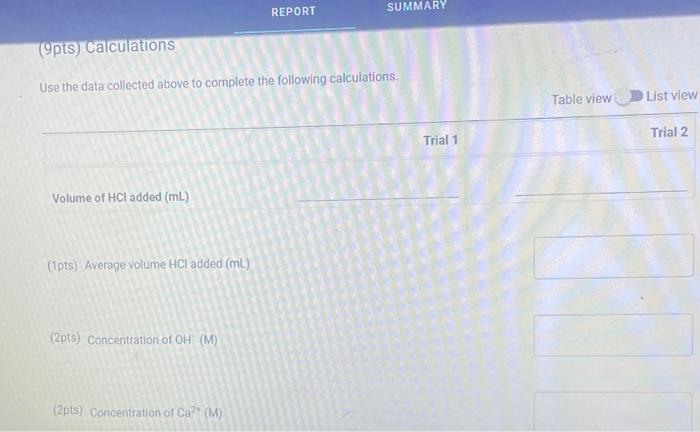
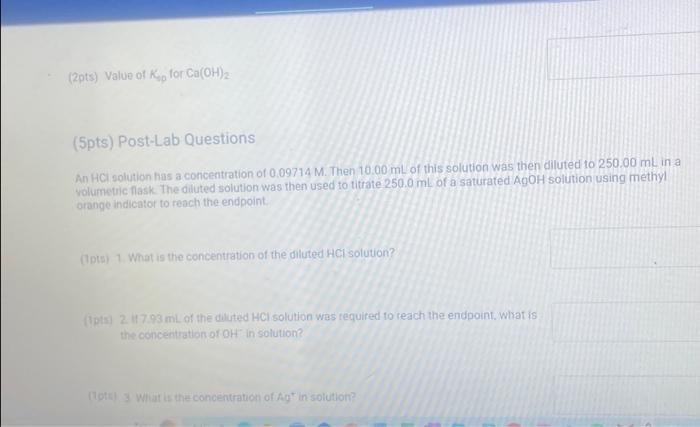
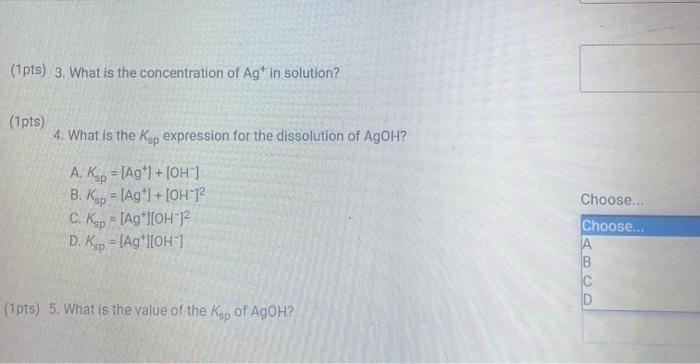
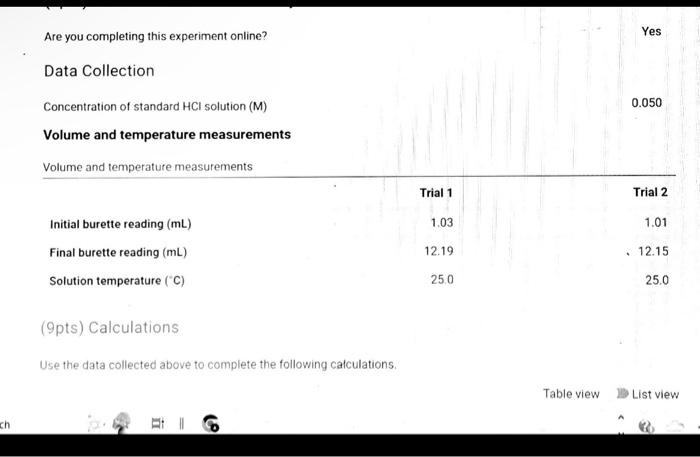
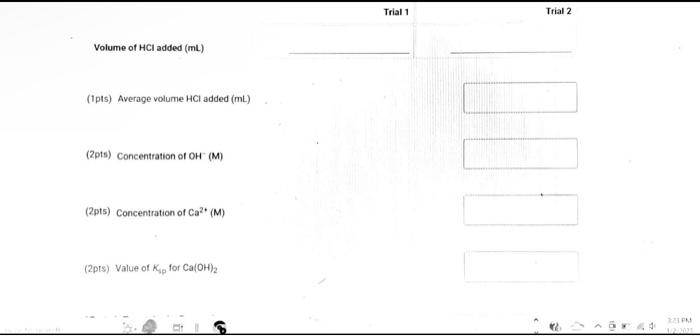
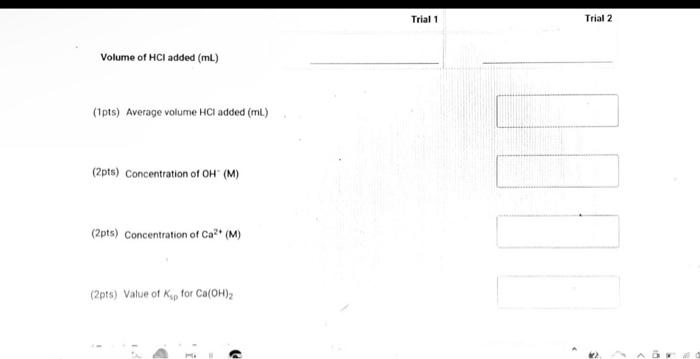
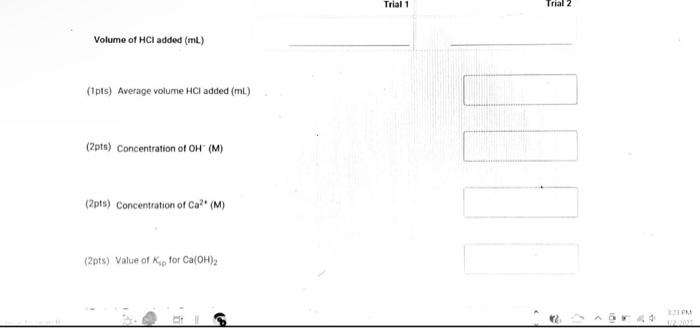
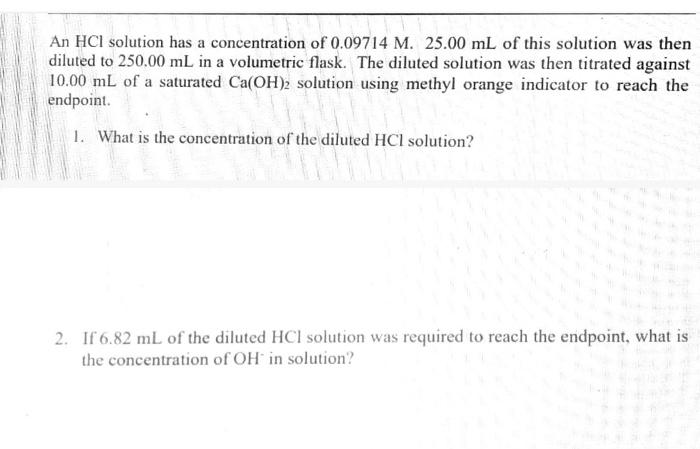
(9pts) Calculations Use the data collected abowe to complete the following caiculations: Use the data collected above to complete the following calculations. (2pts) Value of Ksp for Ca(OH)2 (5pts) Post-Lab Questions An HCi solution has a concentration of 0.09714M. Then 10.00mL of this solution was then diluted to 250.00mL in a volumetic flask. The diluted solution was then used to titrate 250.0mL of a saturated AgOH solution using methyl orange indicator to reach the endpoint. (1pts) 1 What is the concentration of the diluted HCl solution? (Ipts) 2.117.93mL of the dilited HCl solution was tequired to reach the endpoint. What is the concentration of OH " in solution? [10ts) 3 What is the concentration of Ag+in solution? (1pts) 3. What is the concentration of Ag+in solution? (1pts) 4. What is the Ksp expression for the dissolution of AgOH ? A. Ksp=[Ag+]+[OH] B. Kop=[Ag+]+[OH]2 C. Ksp=[Ag+][OH]2 D. Ksp=[Ag+][OH] (1pts) 5. What is the value of the Ksp of AgOH ? (9pts) Calculations Use the data collected above to complete the following calculations. Trial 1 Trial 2 Volume of HCl added (mL) (1pts) Average volume HCl added (mL) (2pts) Concentration of OH(M) (2pts) Concentration of Ca2(M) (2pts) Value of sp for Ca(OH)2 (5pts) Post-Lab Questions An HCl solution has a concentration of 0.09714M. Then 10.00mL of this solution was then diluted to 250.00mL in a volumetric flask. The diluted solution was then used to titrate 250.0mL of a saturated AgOH solution using methyl orange indicator to reach the endpoint. (1pts) 1. What is the concentration of the diluted HCl solution? (1pts) 2. If 7.93mL of the diluted HCl solution was required to reach the endpoint, what is the concentration of OHin solution? (1pts) 3. What is the concentration of Ag+in solution? (1pts) 4. What is the Ksp expression for the dissolution of AgOH ? A Ksp=[Ag+]+[OH] B. K=Aa++[OH]2 Chonse (1pts) 3. What is the concentration of Ag+in solution? (1pts) 4. What is the Ksp expression for the dissolution of AgOH ? A. Ksp=[Ag+]+[OH] B. Ksp=[Ag4]+[OH]2 C. Ksp=AggOH]2 D. Ksp=[Ag][OH] (1pts) 5. What is the value of the K5p of AgOH ? (9pts) Calculations Use the data collected above to complete the following calculations. Volume of HCl added (mL) (Tpts) Average volume HCl added (mL) (2pts) Concentration of OH(M) (2pts) Concentration of Ca2+(M) (2pts) Value of Ksp for Ca(OH)2 Trial 1 Trial 2 Volume of HCl added (mL) (1pts) Average volume HCl added (mL) (2pts) Concentration of OH(M) (2pts) Concentration of Ca2(M) (2pts) Value of sp for Ca(OH)2 An HCl solution has a concentration of 0.09714M. Then 10.00mL of this solution was then diluted to 250.00mL in a volumetric flask. The diluted solution was then used to titrate 250.0mL of a saturated AgOH solution using methyl orange indicator to reach the endpoint. (1pts) 1 . What is the concentration of the diluted HCl solution? (1pts) 2. If 7.93mL of the diluted HCl solution was required to reach the endpoint, what is the concentration of OHin solution? (1pts) 3. What is the concentration of Ag+in solution? (1pts) 4. What is the KSp expression for the dissolution of AgOH ? AKsp=[Ag+]+[OH]B.K=[Aa+]+[OH12 Chonse (1pts) 3. What is the concentration of Ag+in solution? (1pts) 4. What is the Ksp expression for the dissolution of AgOH ? A. Ksp=[Ag+]+[OH] B. Ksp=Ag]+[OH]2 C. Ksp=AgOHH2 D. Ksp=[Ag][OH] Choose... \begin{tabular}{|l|} \hline Choose... \\ \hline B \\ B \\ C \\ D \\ \hline \end{tabular} (1pts) 5. What is the value of the Ksp of AgOH ? An HCl solution has a concentration of 0.09714M.25.00mL of this solution was then diluted to 250.00mL in a volumetric flask. The diluted solution was then titrated against 10.00mL of a saturated Ca(OH)2 solution using methyl orange indicator to reach the endpoint. 1. What is the concentration of the diluted HCl solution? 2. If 6.82mL of the diluted HCl solution was required to reach the endpoint, what is the concentration of OHin solution