Answered step by step
Verified Expert Solution
Question
1 Approved Answer
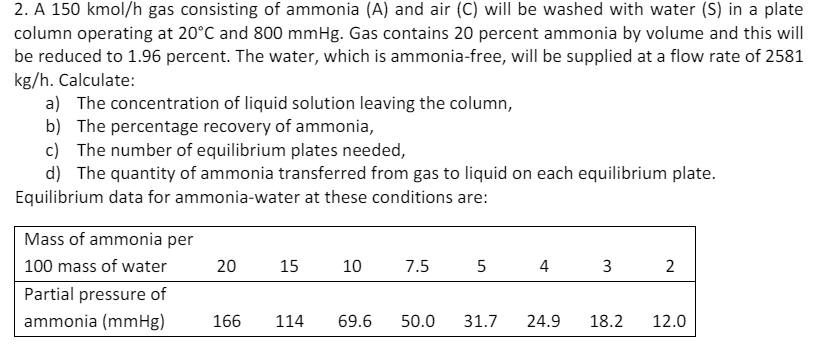
A 1 5 0 kmo l h gas consisting of ammonia ( A ) and air ( C ) will be washed with water (
A kmo gas consisting of ammonia A and air C will be washed with water S in a plate
column operating at and Gas contains percent ammonia by volume and this will
be reduced to percent. The water, which is ammoniafree, will be supplied at a flow rate of
Calculate:
a The concentration of liquid solution leaving the column,
b The percentage recovery of ammonia,
c The number of equilibrium plates needed,
d The quantity of ammonia transferred from gas to liquid on each equilibrium plate.
Equilibrium data for ammoniawater at these conditions are:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


