Question
2 A 1 m cubical-shaped vacuum chamber contains O molecules at a pressure of 104 atm at 300 K. (a) How many molecules are
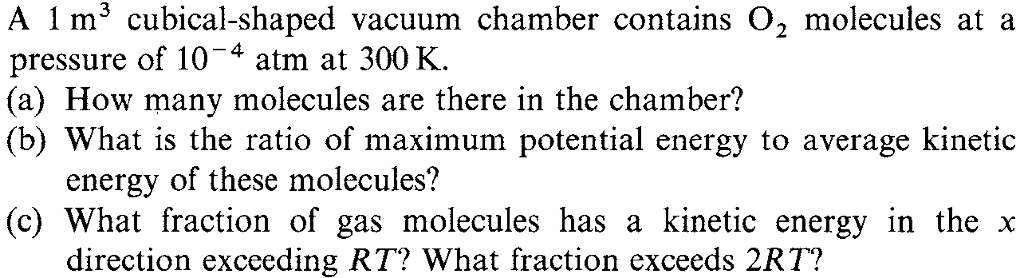
2 A 1 m cubical-shaped vacuum chamber contains O molecules at a pressure of 104 atm at 300 K. (a) How many molecules are there in the chamber? (b) What is the ratio of maximum potential energy to average kinetic energy of these molecules? (c) What fraction of gas molecules has a kinetic energy in the x direction exceeding RT? What fraction exceeds 2RT?
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
University Physics with Modern Physics
Authors: Hugh D. Young, Roger A. Freedman, Lewis Ford
12th Edition
978-0321501479, 9780805321876, 321501470, 978-0321501219
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



