Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 10.0 L scuba diving tank is to be filled with compressed air. The density of air can be approximated using the ideal gas
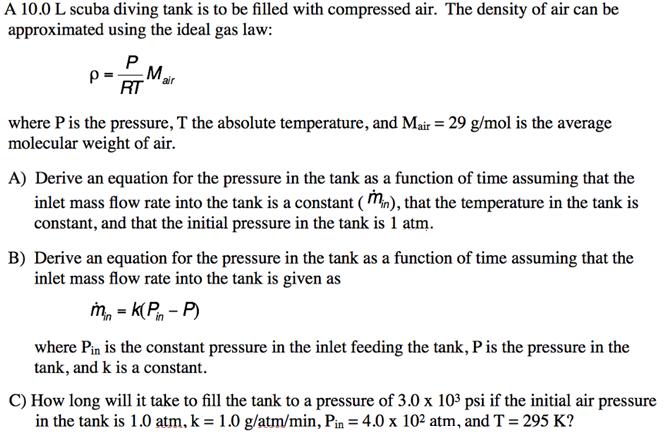
A 10.0 L scuba diving tank is to be filled with compressed air. The density of air can be approximated using the ideal gas law: RT where Pis the pressure, T the absolute temperature, and Mair = 29 g/mol is the average molecular weight of air. A) Derive an equation for the pressure in the tank as a function of time assuming that the inlet mass flow rate into the tank is a constant ( Mn), that the temperature in the tank is constant, and that the initial pressure in the tank is 1 atm. B) Derive an equation for the pressure in the tank as a function of time assuming that the inlet mass flow rate into the tank is given as m, = k(P - P) %3D where Pin is the constant pressure in the inlet feeding the tank, Pis the pressure in the tank, and k is a constant. C) How long will it take to fill the tank to a pressure of 3.0 x 103 psi if the initial air pressure in the tank is 1.0 atm, k = 1.0 g/atm/min, Pin = 4.0 x 102 atm, and T = 295 K?
Step by Step Solution
★★★★★
3.54 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


