Question
A 12.13 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 21.63 grams of CO2 and
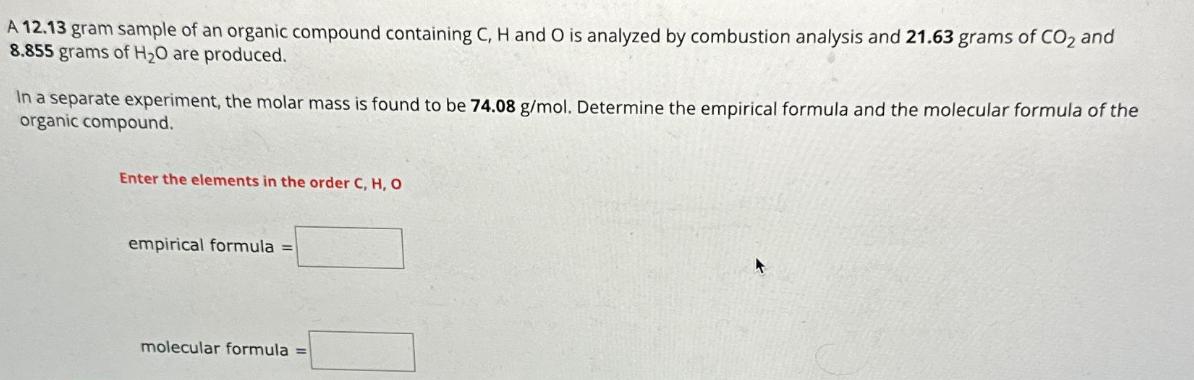
A 12.13 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 21.63 grams of CO2 and 8.855 grams of HO are produced. In a separate experiment, the molar mass is found to be 74.08 g/mol. Determine the empirical formula and the molecular formula of the organic compound. Enter the elements in the order C, H, O empirical formula = molecular formula =
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
To find the empirical formula of the organic compound we will start by analyzing the combustion products to determine the number of moles of carbon C ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Analytical Chemistry
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
9th edition
495558281, 978-0495558286
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



