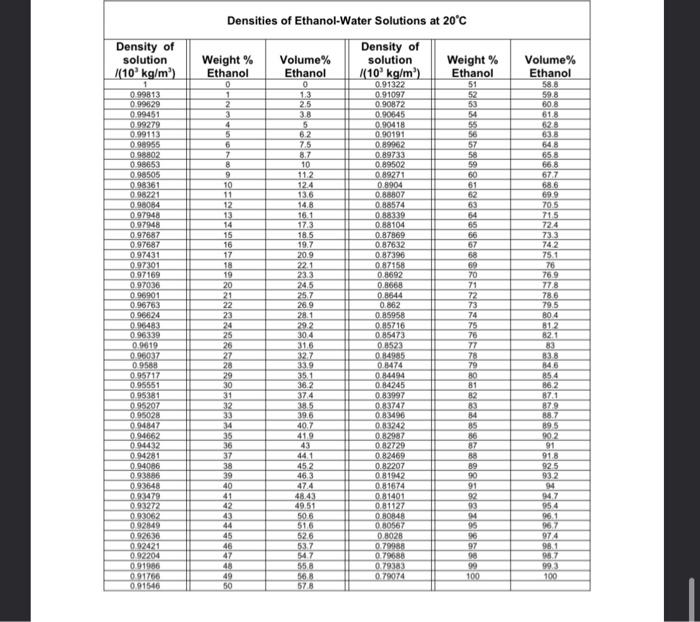
a 15. Penicillin is recovered from an aqueous stream (Aco = 1000kg/m) in a solvent extractor mixer-settler unit using MIBK (ex = 797.8 kg/m) as a solvent. For a feed of 25 m/h containing 10 kg/m of penicillin, (a) Determine the volumetric flow rate of solvent required if the final penicillin concentration in the aqueous stream is to be reduced by 90%. (b) If a second mixer settler is available determine the fresh solvent feed needed to reduce the aqueous penicillin stream from the first unit by a further 90%. Note: the equilibrium relationship between the penicillin concentration in the organic (kg/m) and aqueous (kg/m) phases is, KE =20 16. If instead of the cross flow configuration of 15(b) above a counter current flow arrangement was used, in which the exit solvent from the second stage acted as the solvent feed to the first stage, determine the overall solvent usage and compare this usage to that of (a) and (b) above 17. A tonne of 20 wt% aqueous sodium nitrate (NaNO) is concentrated to saturation by evaporation at 373 K. The solution is then cooled to 293 K to form a slurry of NaNO, crystals in a saturated aqueous solution of NaNos. The crystals of sodium nitrate are removed by filtration of the slurry. The wet filter cake consists of 5% by weight of mother liquor and the remainder is NaNO, crystals. The solubility of sodium nitrate is 1.76 kg/kg HO at 373 K, and 0.88 kg/kg H.O at 293K. Calculate, (a) The amount of water evaporated to achieve saturation at 373K (6) The total weight of dry sodium nitrate obtained 18. A tank holds 5000kg of a saturated solution of Naco, at 333K. If 200 kg of sodium carbonate has to be crystallised from this solution, to what temperature should the solution be cooled Temp/K 333 293 kg Na CO/100 kg HO 19. A cylindrical vessel with its axis horizontal, 2 m in diameter and 6 m in length, and flat ends is slowly filled with benzene (p = 879.0 kg/m). 0 Express the weight (newtons) of the benzene in the tank as a function of the maximum depth of benzene in the tank at any instant. (0) Prepare a plot of the benzene weight as a function of the maximum depth of benzene in the tank. 323 14.5 313 12.7 303 11.1 16.4 9.6 Density of solution (10 kg/m) Volume% Ethanol Densities of Ethanol-Water Solutions at 20C Density of Weight % Volume% solution Weight % Ethanol Ethanol (10 kg/m) Ethanol 0 0 0.91322 51 1 13 0.91097 52 2 2.5 0.90872 53 3 38 0.90645 4 5 0.90418 55 5 62 0.90191 56 6 75 0.89962 57 7 8.7 0.89733 58 8 10 0.89502 59 9 112 0.89271 60 10 124 0.8904 61 11 13.6 0.88807 62 12 14.8 0.88574 63 13 16.1 0.88339 64 14 17.3 0.88104 65 15 185 0.87869 66 16 19.7 0.87632 67 17 20.9 0.87396 68 18 221 0.87158 69 19 23.3 0.8692 70 20 24,5 0.8668 71 21 257 0.8644 72 22 28.9 0.862 73 23 28.1 0.85958 74 24 29.2 0.85716 75 25 304 0.85473 76 26 31.5 0.1523 77 27 32.7 0.84985 78 28 339 0.8474 79 29 351 0.84494 80 30 36.2 0.84245 81 31 374 0.83997 82 32 38.5 0.83747 33 39.6 0.83496 84 34 40.7 0.83242 85 35 41.9 0.82987 86 36 0.82729 87 37 441 0.82469 88 38 452 0.82207 89 39 463 081942 90 40 47.4 081674 91 41 4843 0.81401 92 42 49.51 081127 33 43 505 080848 94 44 51.6 080567 95 45 526 08028 96 46 537 0.79988 97 47 547 0.79688 98 48 958 0.79383 99 49 36,8 0.790974 100 50 578 0.99813 0.99629 0.99451 099279 0.99113 098955 098802 0.98653 0.98505 098361 098221 9.98084 0.97948 0.979448 9.97687 0.97687 0.97431 0.97301 0.97169 0.97036 096901 0.96763 0.96624 0.95483 0.96339 0.9619 0.00037 09588 0.95717 0.95551 0.95381 0.95207 0.95028 0.94347 0.94662 0.94432 0.94281 0.94086 0.93886 093548 0.93479 0.93272 0.93062 092849 092636 0.92421 092204 091986 0.91766 0.91546 598 80.8 618 628 638 548 658 668 677 68.6 699 705 715 724 73.3 742 75.1 76 76.9 778 786 79.5 B04 812 82.1 838 84.6 85.4 862 871 879 88.7 895 90.2 91 91.8 925 932 94 947 954 96.1 967 974 98.1 987 993 100