Answered step by step
Verified Expert Solution
Question
1 Approved Answer
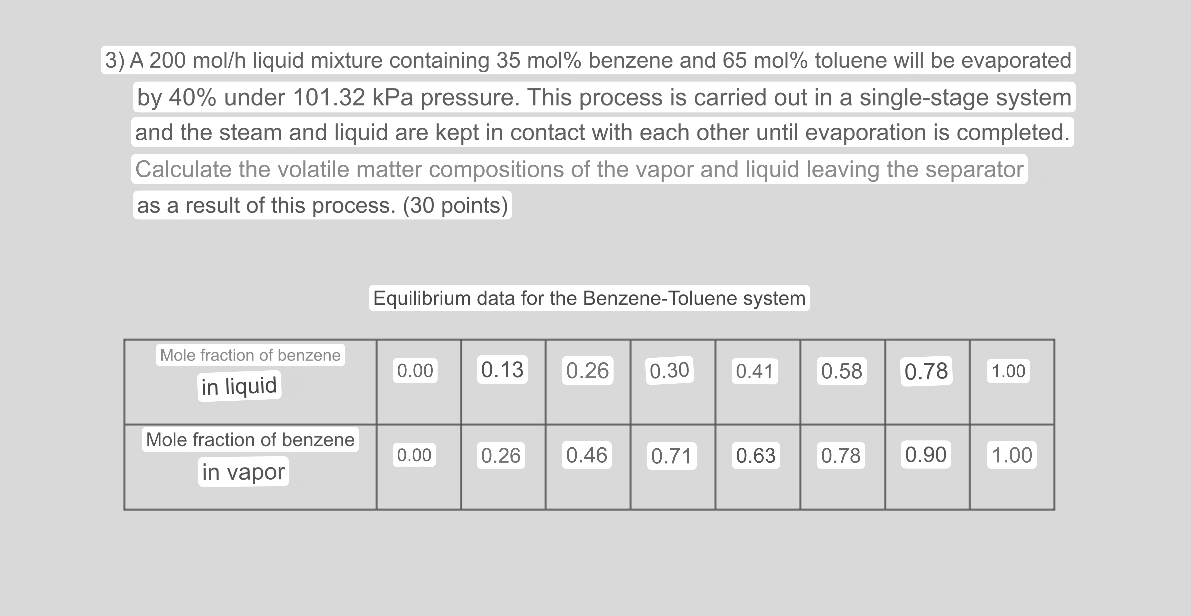
A 2 0 0 m o l h liquid mixture containing 3 5 mol % benzene and 6 5 mol % toluene will be evaporated
A liquid mixture containing mol benzene and mol toluene will be evaporated by under kPa pressure. This process is carried out in a singlestage system and the steam and liquid are kept in contact with each other until evaporation is completed.
Calculate the volatile matter compositions of the vapor and liquid leaving the separator as a result of this process. points
Equilibrium data for the BenzeneToluene system
tabletableMole fraction of benzenein liquidtableMole fraction of benzenein vapor
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


