Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 21.3 gram sample of poly(hexamethylene adipamide) is found to contain 2.50 x 10-3 moles of carboxyl groups by both titration and infrared spectroscopy. Assume
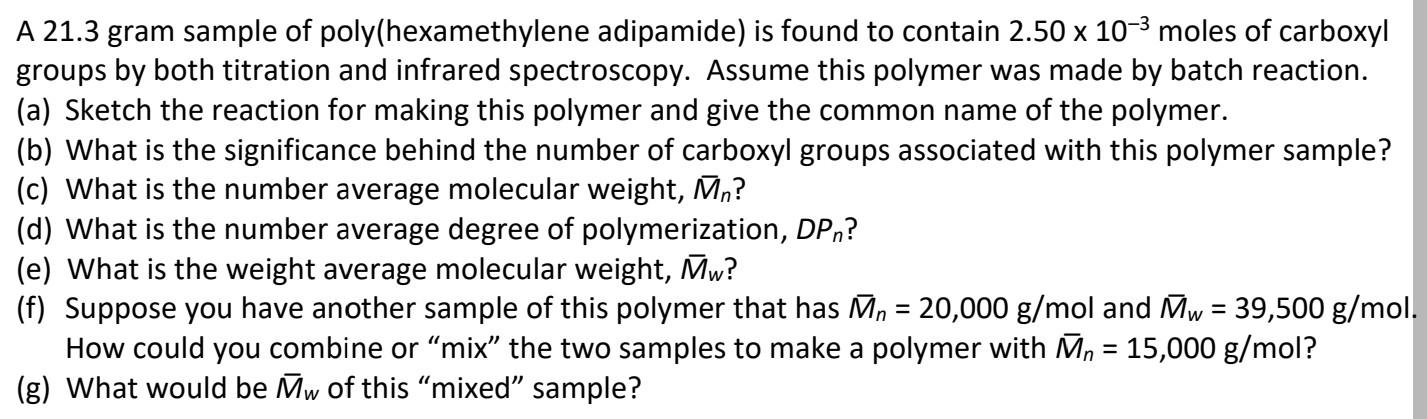
A 21.3 gram sample of poly(hexamethylene adipamide) is found to contain 2.50 x 10-3 moles of carboxyl groups by both titration and infrared spectroscopy. Assume this polymer was made by batch reaction. (a) Sketch the reaction for making this polymer and give the common name of the polymer. (b) What is the significance behind the number of carboxyl groups associated with this polymer sample? (c) What is the number average molecular weight, Mn? (d) What is the number average degree of polymerization, DP,? (e) What is the weight average molecular weight, Mw? (f) Suppose you have another sample of this polymer that has Mn = 20,000 g/mol and Mw = 39,500 g/mol. How could you combine or mix the two samples to make a polymer with Mn = 15,000 g/mol? (g) What would be Mw of this mixed sample? = A 21.3 gram sample of poly(hexamethylene adipamide) is found to contain 2.50 x 10-3 moles of carboxyl groups by both titration and infrared spectroscopy. Assume this polymer was made by batch reaction. (a) Sketch the reaction for making this polymer and give the common name of the polymer. (b) What is the significance behind the number of carboxyl groups associated with this polymer sample? (c) What is the number average molecular weight, Mn? (d) What is the number average degree of polymerization, DP,? (e) What is the weight average molecular weight, Mw? (f) Suppose you have another sample of this polymer that has Mn = 20,000 g/mol and Mw = 39,500 g/mol. How could you combine or mix the two samples to make a polymer with Mn = 15,000 g/mol? (g) What would be Mw of this mixed sample? =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


