Question
A 25-mm hole occurs in a large pipeline resulting in a leak of pure methane gas and a flame. The methane is at a pressure
A 25-mm hole occurs in a large pipeline resulting in a leak of pure methane gas and a flame. The methane is at a pressure of 100 bar gauge. The leak occurs 2-m off the ground. Determine: i) The height of the flame using below equation
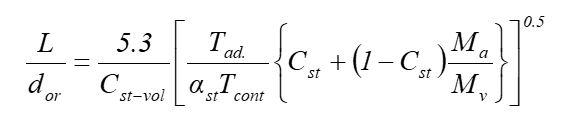
ii) The discharge rate (kg/s), and iii) The radiant heat flux at a point on the ground 15 m from the resulting flame.
Additional Data:
The ambient temperature: 298 K
The humidity: 50% RH.
The discharge coefficient: 1.0
Heat capacity ratio, , for methane: 1.32
Heat of combustion for methane: 50,000 kJ/kg
Flame temperature for methane: 2200 K
Molecular weight for air: 29
Molecular weight for methane: 16
dorL=Cstvol5.3[stTcontTad{Cst+(1Cst)MvMa}]0.5Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


