Answered step by step
Verified Expert Solution
Question
1 Approved Answer
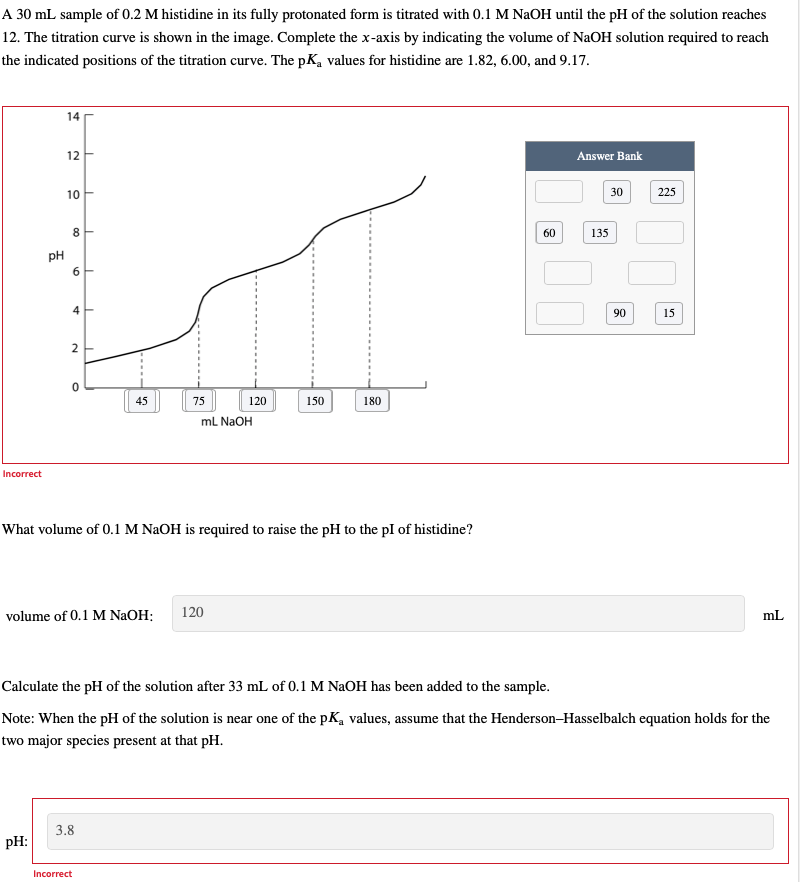
A 3 0 m L sample of 0 . 2 M histidine in its fully protonated form is titrated with 0 . 1 MNaOH until
A sample of histidine in its fully protonated form is titrated with MNaOH until the of the solution reaches
The titration curve is shown in the image. Complete the axis by indicating the volume of NaOH solution required to reach
the indicated positions of the titration curve. The values for histidine are and
Answer Bank
Incorrect
What volume of MNaOH is required to raise the to the of histidine?
volume of MNaOH :
Calculate the of the solution after of MNaOH has been added to the sample.
Note: When the of the solution is near one of the values, assume that the HendersonHasselbalch equation holds for the
two major species present at that
:
Incorrect
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


