Answered step by step
Verified Expert Solution
Question
1 Approved Answer
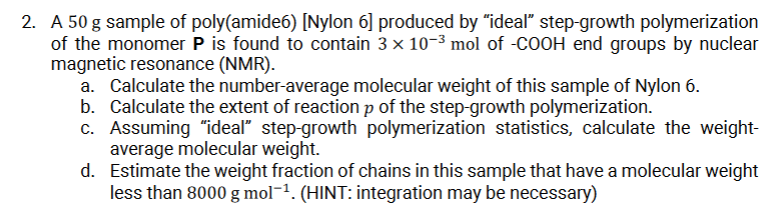
A 5 0 g sample of poly ( amide 6 ) [ Nylon 6 ] produced by ideal step - growth polymerization of the monomer
A sample of polyamideNylon produced by "ideal" stepgrowth polymerization
of the monomer is found to contain mol of end groups by nuclear
magnetic resonance NMR
a Calculate the numberaverage molecular weight of this sample of Nylon
b Calculate the extent of reaction of the stepgrowth polymerization.
c Assuming "ideal" stepgrowth polymerization statistics, calculate the weight
average molecular weight.
d Estimate the weight fraction of chains in this sample that have a molecular weight
less than HINT: integration may be necessary Molar mass of P is gmol It is CHON
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


