Question
A 5 m^3 cylinder containing nitrogen gas initially at a pressure of 200 bar and 255K is connected to another cylinder 0.05 m^3 in volume,
A 5 m^3 cylinder containing nitrogen gas initially at a pressure of 200 bar and 255K is connected to another cylinder 0.05 m^3 in volume, which is initially evacuated. A valve between the two cylinders is opened until the pressures in the cylinders equalize. Find the final temperature and pressure in each cylinder if there is no heat flow into or out of the cylinder. You may assume that there is no heat transfer between the gas and the cylinder walls and that the gas is ideal with a constant pressure heat capacity of 28 J/mol-K.
Answer all questions,if you can't don't attempt the answer . And this is the complete question.
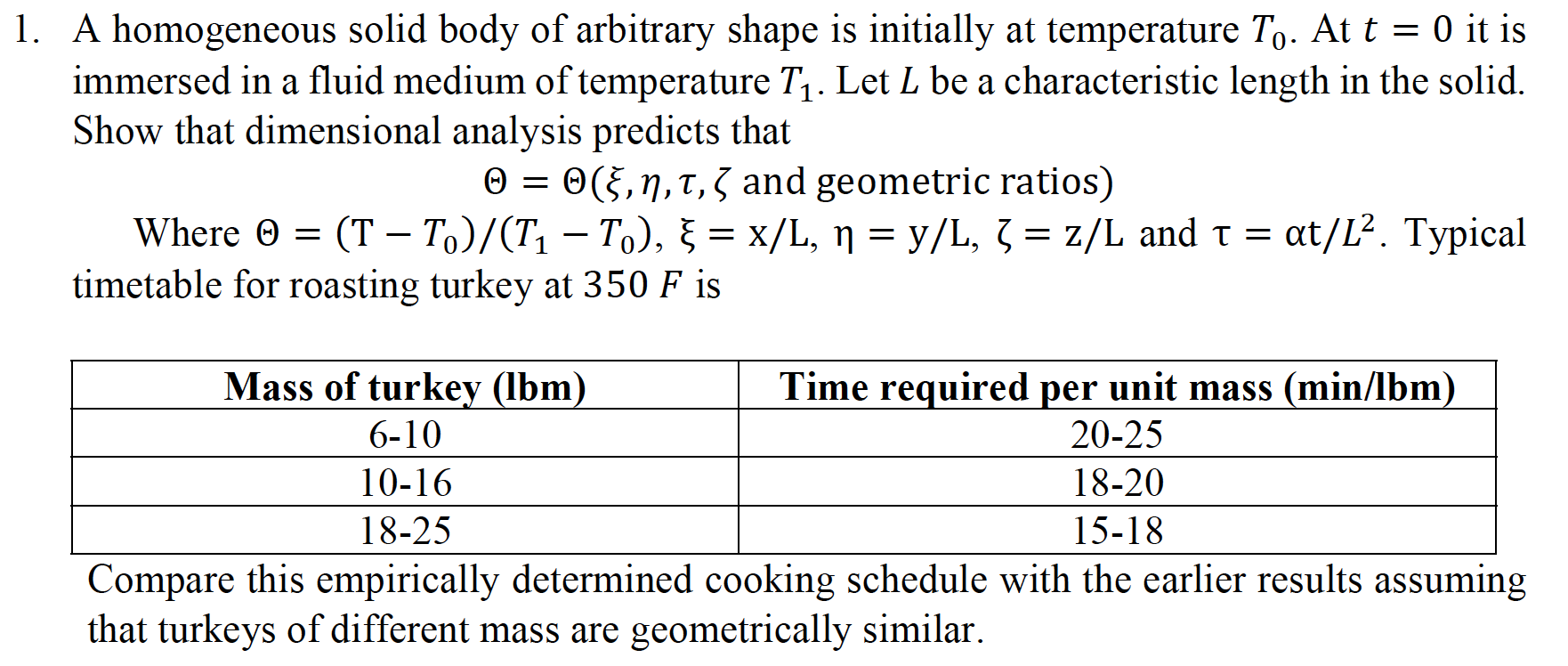
A homogeneous solid body of arbitrary shape is initially at temperature T0. At t=0 it is immersed in a fluid medium of temperature T1. Let L be a characteristic length in the solid. Show that dimensional analysis predicts that =(,,,andgeometricratios) Where =(TT0)/(T1T0),=x/L,=y/L,=z/L and =t/L2. Typical timetable for roasting turkey at 350F is Compare this empirically determined cooking schedule with the earlier results assuming that turkeys of different mass are geometrically similar
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


