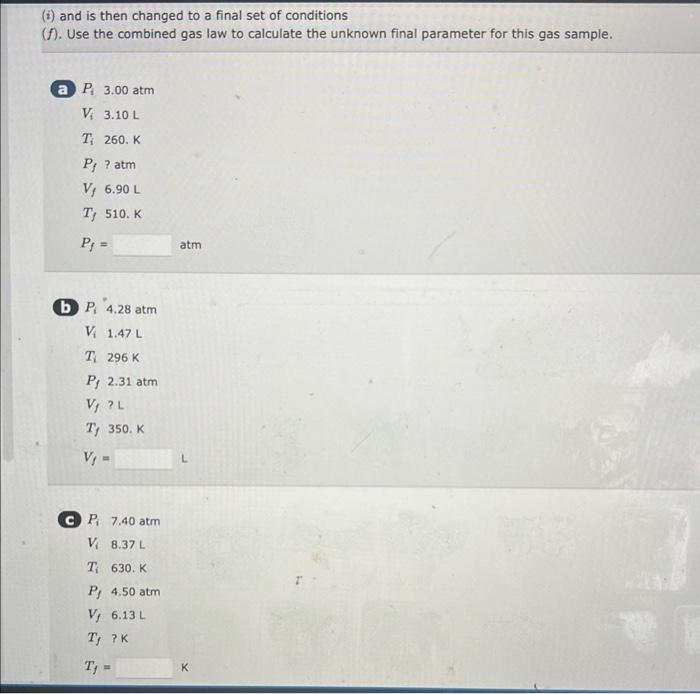
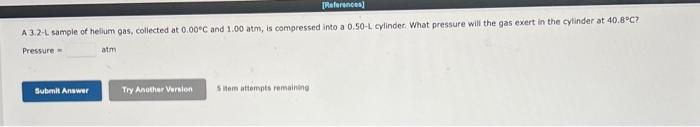
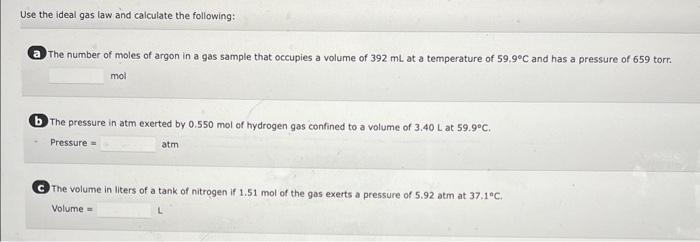
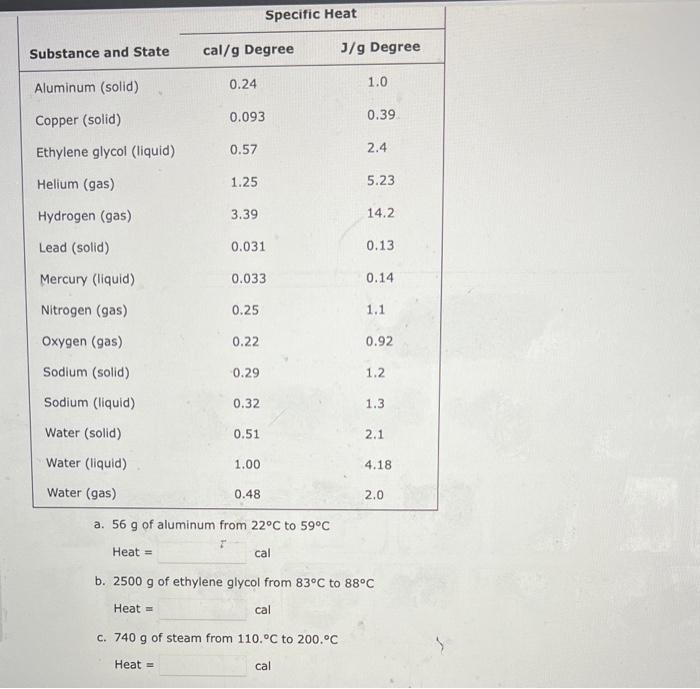
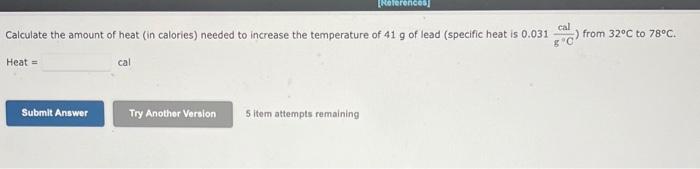
A 6.76L sample of gas has a pressure of 1.72 atm and a temperature of 88 .C. The sample is compressed to a volume of 4.21L and is heated to 140 . C. Calculate the new pressure of the gas, assuming that no gas escaped during the experiment. atm A balloon holds 32.2kg of helium. What is the volume of the balloon if the pressure is 1.1atm and the temperature is 24C ? L (i) and is then changed to a final set of conditions (f). Use the combined gas law to calculate the unknown final parameter for this gas sample. Pi3.00atm Vi3.10L Ti260.K Pf?atm Vf6.90L Tf510.K Pf= (b) Pi4.28atm Vi1.47L Ti296K Pf2.31atm Vf?L Tf350.K Vf= C Pi7.40atm Vi8.37L Ti630.K Pf4.50atm Vf6.13L Tf?K Tf= A 3.2-L sample of helium gas, collected at 0.00C and 1.00 atm, is compressed into a 0.501 cylindec. What pressure will the gas exert in the cytinder at 40.8C ? Pressure=atm Use the ideal gas law and calculate the following: a The number of moles of argon in a gas sample that occupies a volume of 392mL at a temperature of 59.9C and has a pressure of 659 torr. mol (b) The pressure in atm exerted by 0.550 mol of hydrogen gas confined to a volume of 3.40L at 59.99C. -Pressure= C. The volume in liters of a tank of nitrogen if 1.51 mol of the gas exerts a pressure of 5.92 atm at 37.1C. Volume = Calculate the volume occupied by 5.35g of oxygen gas (O2) at a pressure of 0.692 atm and a temperature of 42.2C. Volume = 5 item attempts remaining A steel cylinder contains a mixture of nitrogen, oxygen, and carbon dioxide gases. The total pressure in the tank is 1280 torr. The pressure exerted by the nitrogen and oxygen is 530 and 310 torr, respectively. What is the partial pressure in torr of the carbon dioxide in the mixture? (Enter your answer to two significant figures.) PCO3=torr a. 56g of aluminum from 22C to 59C Heat = b. 2500g of ethylene glycol from 83C to 88C Heat = cal C. 740g of steam from 110.C to 200.C Heat =cal Calculate the amount of heat (in calories) needed to increase the temperature of 41g of lead (specific heat is 0.031gCcal ) from 32C to 78C. Heat =cal