Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a. A pressure difference of 1000kPa would cause the same flux of N2 through polypropylene as a pressure difference of 100kPa would cause for O2
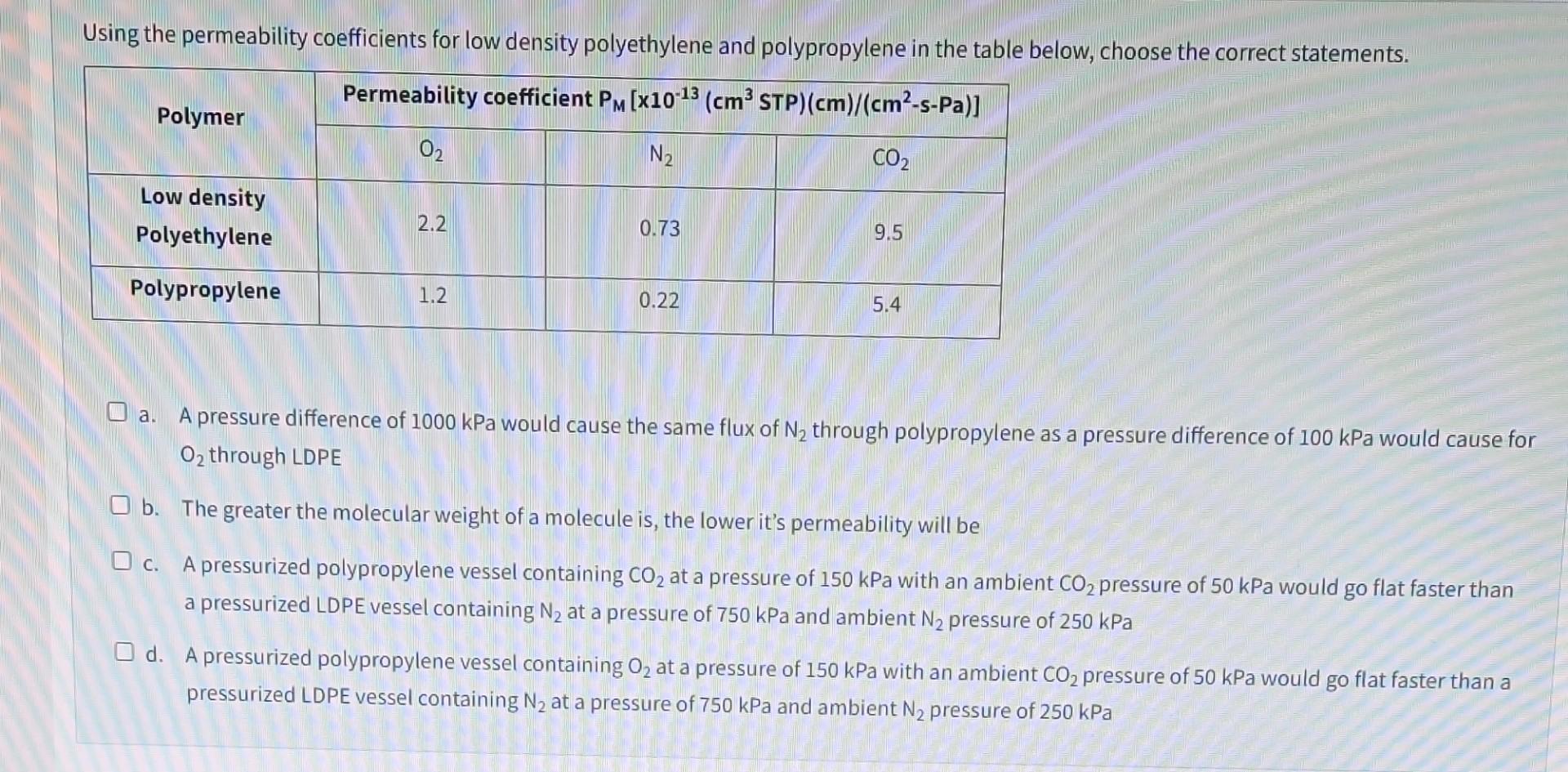
a. A pressure difference of 1000kPa would cause the same flux of N2 through polypropylene as a pressure difference of 100kPa would cause for O2 through LDPE b. The greater the molecular weight of a molecule is, the lower it's permeability will be c. A pressurized polypropylene vessel containing CO2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa d. A pressurized polypropylene vessel containing O2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa a. A pressure difference of 1000kPa would cause the same flux of N2 through polypropylene as a pressure difference of 100kPa would cause for O2 through LDPE b. The greater the molecular weight of a molecule is, the lower it's permeability will be c. A pressurized polypropylene vessel containing CO2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa d. A pressurized polypropylene vessel containing O2 at a pressure of 150kPa with an ambient CO2 pressure of 50kPa would go flat faster than a pressurized LDPE vessel containing N2 at a pressure of 750kPa and ambient N2 pressure of 250kPa
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


