Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) Assume you are evaporating Gold in a vacuum chamber of dimension 1 m x 1 mx 1 m. Residual air present after evacuating
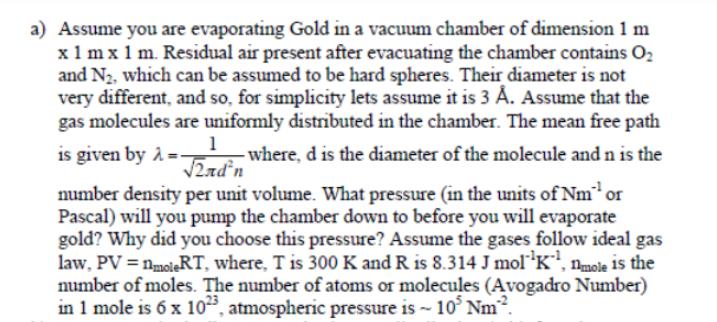
a) Assume you are evaporating Gold in a vacuum chamber of dimension 1 m x 1 mx 1 m. Residual air present after evacuating the chamber contains O and N, which can be assumed to be hard spheres. Their diameter is not very different, and so, for simplicity lets assume it is 3 A. Assume that the gas molecules are uniformly distributed in the chamber. The mean free path 1 is given by A-- - where, d is the diameter of the molecule and n is the 2ndn number density per unit volume. What pressure (in the units of Nm or Pascal) will you pump the chamber down to before you will evaporate gold? Why did you choose this pressure? Assume the gases follow ideal gas law, PV = nmoleRT, where, T is 300 K and R is 8.314 J molK, mole is the number of moles. The number of atoms or molecules (Avogadro Number) in 1 mole is 6 x 1023, atmospheric pressure is ~ 10 Nm.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Calculation of Pressure Required to Evaporate Gold in a Vacuum Chamber To determine the pressure req...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


