Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) At a temperature T=3 x 10 K, and assuming the B-decays in the CN cycle are fast enough to be considered effectively instantaneous, determine
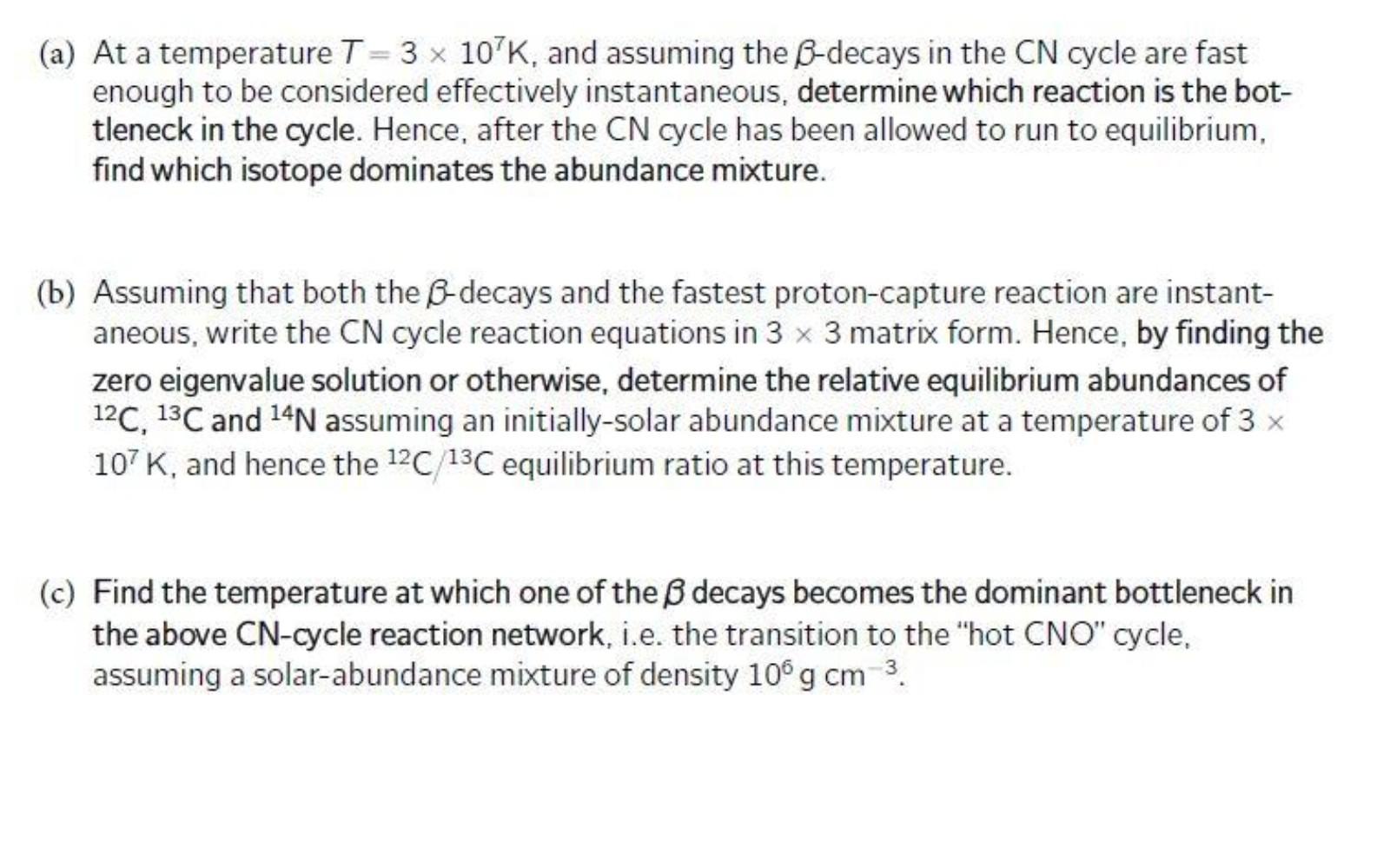
(a) At a temperature T=3 x 10 K, and assuming the B-decays in the CN cycle are fast enough to be considered effectively instantaneous, determine which reaction is the bot- tleneck in the cycle. Hence, after the CN cycle has been allowed to run to equilibrium, find which isotope dominates the abundance mixture. (b) Assuming that both the B-decays and the fastest proton-capture reaction are instant- aneous, write the CN cycle reaction equations in 3 x 3 matrix form. Hence, by finding the zero eigenvalue solution or otherwise, determine the relative equilibrium abundances of 12C, 13C and 14N assuming an initially-solar abundance mixture at a temperature of 3 x 107 K, and hence the 12C 13C equilibrium ratio at this temperature. (c) Find the temperature at which one of the decays becomes the dominant bottleneck in the above CN-cycle reaction network, i.e. the transition to the "hot CNO" cycle, assuming a solar-abundance mixture of density 10 g cm 3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


