Answered step by step
Verified Expert Solution
Question
1 Approved Answer
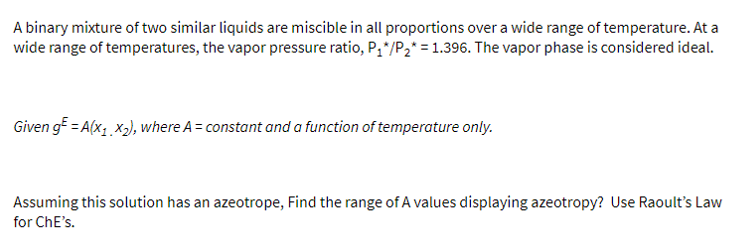
A binary mixture of two similar liquids are miscible in all proportions over a wide range of temperature. At a wide range of temperatures, the
A binary mixture of two similar liquids are miscible in all proportions over a wide range of temperature. At a
wide range of temperatures, the vapor pressure ratio, The vapor phase is considered ideal.
Given where constant and a function of temperature only.
Assuming this solution has an azeotrope, Find the range of A values displaying azeotropy? Use Raoult's Law
for ChE's.
Don't copy from chegg or any other websites please, do it on your own.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


