Answered step by step
Verified Expert Solution
Question
1 Approved Answer
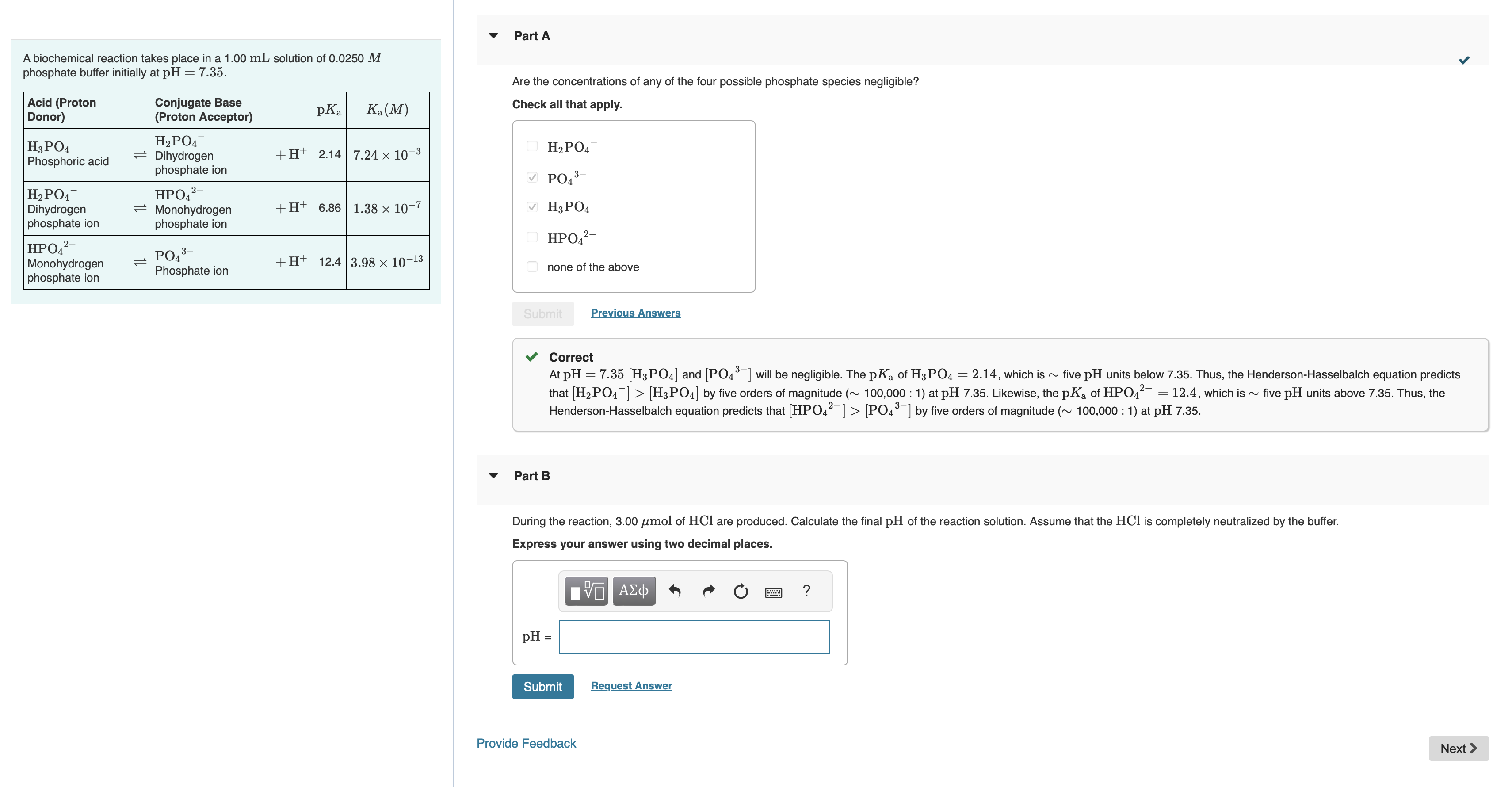
A biochemical reaction takes place in a 1 . 0 0 m L solution of 0 . 0 2 5 0 M phosphate buffer initially
A biochemical reaction takes place in a solution of
phosphate buffer initially at
Part A
Are the concentrations of any of the four possible phosphate species negligible?
Check all that apply.
none of the above
Previous Answers
Correct
At and will be negligible. The of which is five units below Thus, the HendersonHasselbalch equation predicts
that by five orders of magnitude : at Likewise, the of which is five units above Thus, the
HendersonHasselbalch equation predicts that by five orders of magnitude : at
Part B
During the reaction, of are produced. Calculate the final of the reaction solution. Assume that the is completely neutralized by the buffer.
Express your answer using two decimal places.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


