Question
A BTX unit, shown in Figure 4.20, is associated with a refinery that produces benzene, toluene, and xylenes. Stream #1 leaving the reactor-reforming unit has
A BTX unit, shown in Figure 4.20, is associated with a refinery that produces benzene, toluene, and xylenes. Stream #1 leaving the reactor-reforming unit has a volumetric flow rate of 10 m3/h and is a
Figure Reactor and distillation unit 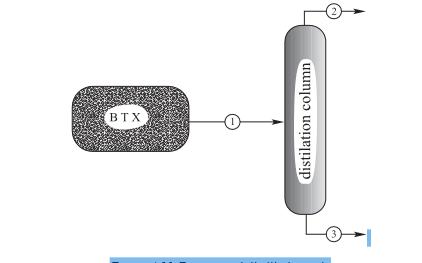
mixture of benzene (A), toluene (B), and xylenes (C) with the following composition: (cA)1= 6,000mol/m , (cA)2=2,000 mol/m , (cA)3=2,000mol/m
Stream (1) is the feed to a distillation unit where the separation takes place according to the following pecifications: s
1. 98% of the benzene leaves with the distillate stream (stream #2).
2. 99% of the toluene in the feed leaves with the bottoms stream (stream #3)
3. 100% of the xylenes in the feed leaves with the bottoms stream (stream #3).
Assuming that the volumes of components are additive, and using the densities of pure components from Table I in the Appendix, compute the concentration and volumetric flow rate of the distillate (stream #2) and bottoms (stream #3) stream leaving the distillation unit.
BTX distilation column
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


