Answered step by step
Verified Expert Solution
Question
1 Approved Answer
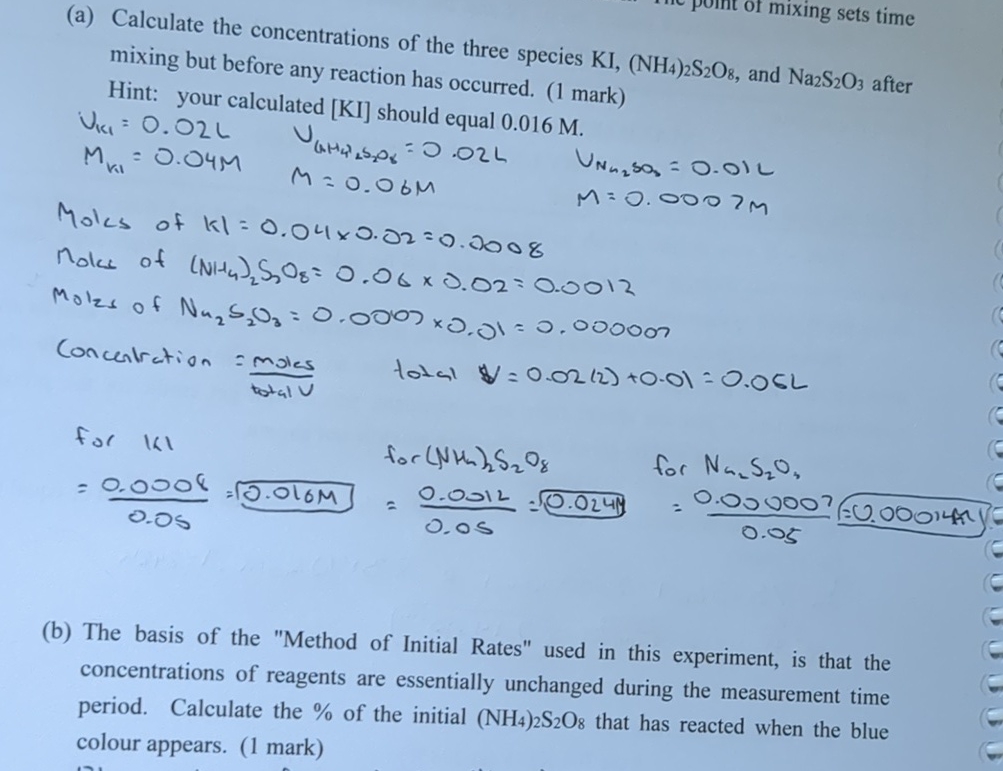
(a) Calculate the concentrations of the three species KI,(NH_(4))_(2)S_(2)O_(8) , and Na_(2)S_(2)O_(3) after mixing but before any reaction has occurred. (1 mark) Hint: your calculated
(a) Calculate the concentrations of the three species
KI,(NH_(4))_(2)S_(2)O_(8), and
Na_(2)S_(2)O_(3)after mixing but before any reaction has occurred. (1 mark)\ Hint: your calculated
KIshould equal
0.016M.\
M_(k1)=0.04M\ M=0.06M\ M=0.0007m\
U_(N_(n_(2))S_(3))=0.01L\ M=0.0007m\ Moles of
Kl=0.04\\\\times 0.02=0.0008\ nolus of
(NH_(4))_(2)S_(3)O_(8)=0.06\\\\times 0.02=0.0012\ Molzs of
\\\\Nu _(2)S_(2)O_(3)=0.0007\\\\times 0.01=0.000007\ Concentration
=( moles )/( totalv ),total
$=0.02(2)+0.01=0.06L\ for
k1\ (b) The basis of the "Method of Initial Rates" used in this experiment, is that the concentrations of reagents are essentially unchanged during the measurement time period. Calculate the
%of the initial
(NH_(4))_(2)S_(2)O_(8)that has reacted when the blue colour appears. (1 mark)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


