Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) Calculate the Debye length (k1) for the amino group of protein lysozyme in 4MNaCl aqueous solution at 25C. (3 marks) (b) The zeta-potential of
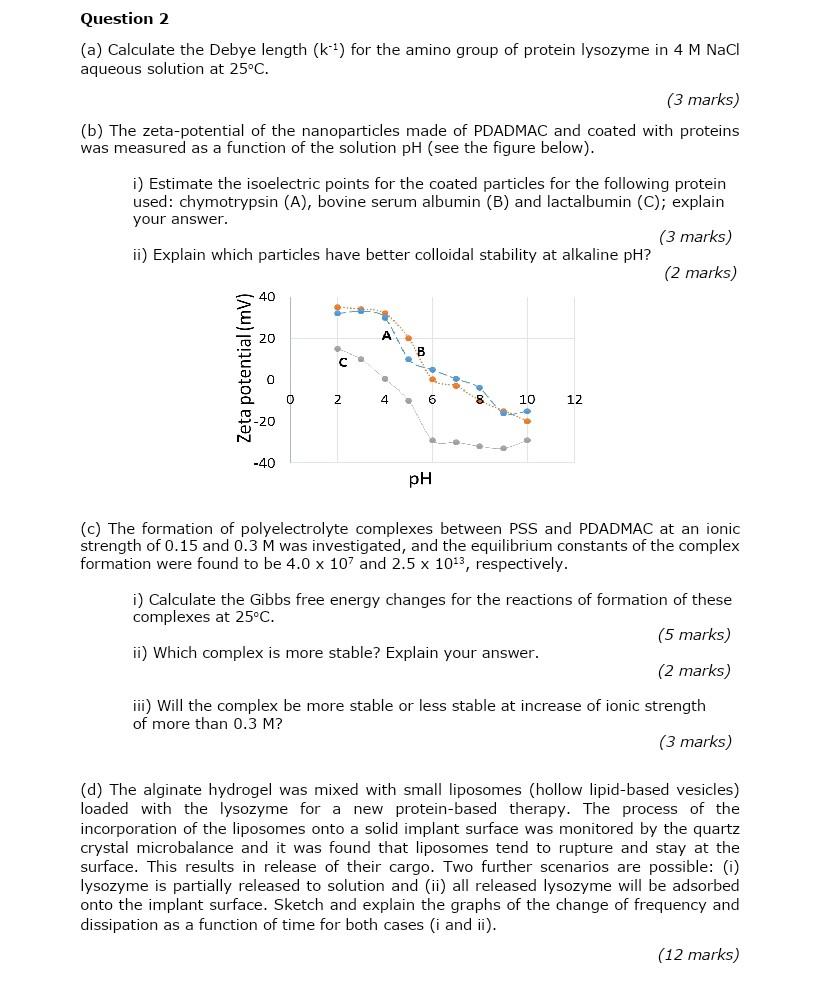
(a) Calculate the Debye length (k1) for the amino group of protein lysozyme in 4MNaCl aqueous solution at 25C. (3 marks) (b) The zeta-potential of the nanoparticles made of PDADMAC and coated with proteins was measured as a function of the solution pH (see the figure below). i) Estimate the isoelectric points for the coated particles for the following protein used: chymotrypsin (A), bovine serum albumin (B) and lactalbumin (C); explain your answer. ii) Explain which particles have better colloidal stability at alkaline pH? (3 marks) (2 marks) (c) The formation of polyelectrolyte complexes between PSS and PDADMAC at an ionic strength of 0.15 and 0.3M was investigated, and the equilibrium constants of the complex formation were found to be 4.0107 and 2.51013, respectively. i) Calculate the Gibbs free energy changes for the reactions of formation of these complexes at 25C. ii) Which complex is more stable? Explain your answer. (5 marks) (2 marks) iii) Will the complex be more stable or less stable at increase of ionic strength of more than 0.3M ? (3 marks) (d) The alginate hydrogel was mixed with small liposomes (hollow lipid-based vesicles) loaded with the lysozyme for a new protein-based therapy. The process of the incorporation of the liposomes onto a solid implant surface was monitored by the quartz crystal microbalance and it was found that liposomes tend to rupture and stay at the surface. This results in release of their cargo. Two further scenarios are possible: (i) lysozyme is partially released to solution and (ii) all released lysozyme will be adsorbed onto the implant surface. Sketch and explain the graphs of the change of frequency and dissipation as a function of time for both cases (i and ii)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


