Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) Calculate the solubility of CaF2 in; (0) Water 0.1M Ca(NO3)2 solution (iii) 0.1M NaF solution Where Ks(CaF2) = 4x10-11 at 25C (b) By referring
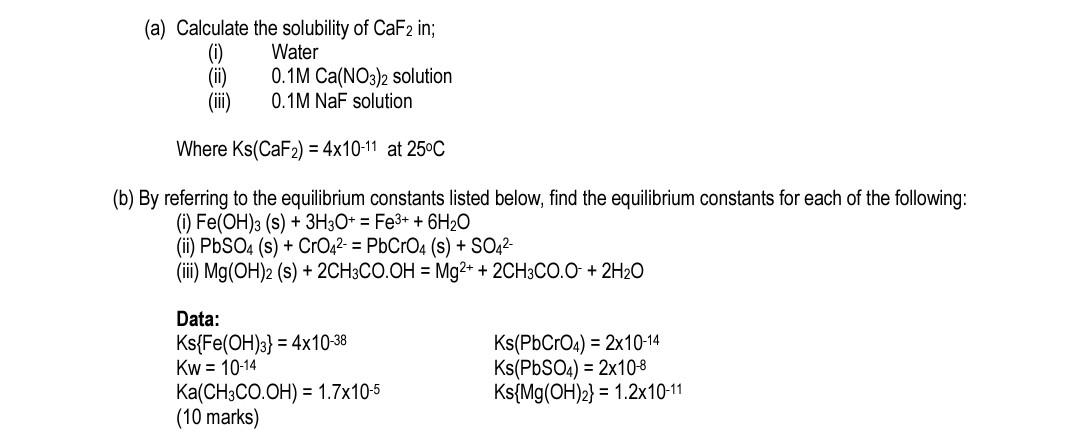
(a) Calculate the solubility of CaF2 in; (0) Water 0.1M Ca(NO3)2 solution (iii) 0.1M NaF solution Where Ks(CaF2) = 4x10-11 at 25C (b) By referring to the equilibrium constants listed below, find the equilibrium constants for each of the following: (0) Fe(OH)3 (s) + 3H3O+ = Fe3+ + 6H20 (ii) PbSO4 (s) + CrO42- = PbCrO4 (s) + SO42- (iii) Mg(OH)2 (s) + 2CH3CO.OH = Mg2+ + 2CH3CO.O + 2H20 Data: Ks{Fe(OH)3} = 4x10-38 Kw = 10-14 Ka(CH3CO.OH) = 1.7x10-5 (10 marks) Ks(PbCrO4) = 2x10-14 Ks(PbSO4) = 2x10-8 Ks{Mg(OH)2) = 1.2x10-11
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


