Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A candidate drug requires an $18 million investment for phase II clinical trials. If the trials are successful (44% probability), the company will learn

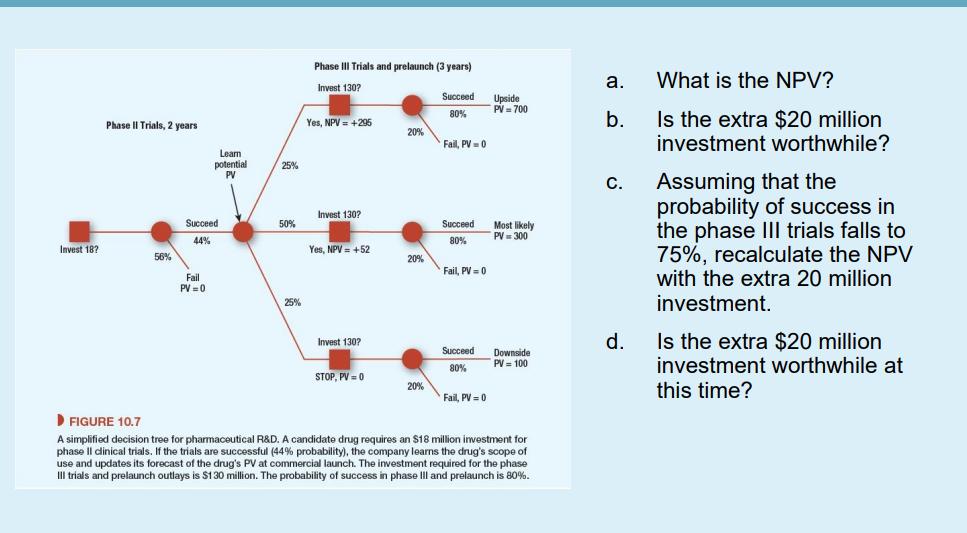
A candidate drug requires an $18 million investment for phase II clinical trials. If the trials are successful (44% probability), the company will learn the drug's scope of use and update its forecast of the drug's PV at commercial launch. The investment required for the phase III trials and prelaunch outlays is $130 million. The probability of success in phase III and prelaunch is 80%. Launch comes three years after the start of phase Ill if the drug is approved by the FDA. The probabilities of the upside, most likely, and downside outcomes are 25%, 50%, and 25%, respectively. If the phase III trials are successful, the manager will learn the commercial potential of the drug, which will depend on how widely it can be used. Suppose that the forecasted PV at launch depends on the scope of use allowed by the FDA. These are an upside outcome of NPV = $700 million if the drug can be widely used, a most likely case with NPV = $300 million, and a downside case of NPV = $100 million if the drug's scope is greatly restricted. We assume a risk-free rate of 4% and market risk premium of 7%. Assume FDA-approved pharmaceutical products have asset betas of 0.8 and the opportunity cost of capital is 9.6%. Accordingly, the NPV works out to $19 million. The R&D team has put forward a proposal to invest an extra $20 million in expanded phase II trials. Phase II takes two years. Th object is to prove that the drug can be administered by a simple inhaler rather than as a liquid. If successful, the scope of use is broadened and the upside PV increases to $1 billion. The probabilities of success are unchanged. Invest 18? Phase II Trials, 2 years 56% Learn potential PV Succeed 44% Fail PV=0 25% 50% 25% Phase Trials and prelaunch Invest 130? Yes, NPV = +295 Invest 130? Yes, NPV +52 Invest 130? STOP, PV = 0 20% 20% 20% years) Succeed 80% Fail, PV = 0 Succeed 80% Fail, PV=0 Succeed 80% Fail, PV = 0 Upside PV = 700 Most likely PV = 300 Downside PV = 100 FIGURE 10.7 A simplified decision tree for pharmaceutical R&D. A candidate drug requires an $18 million investment for phase Il clinical trials. If the trials are successful (44% probability), the company learns the drug's scope of use and updates its forecast of the drug's PV at commercial launch. The investment required for the phase III trials and prelaunch outlays is $130 million. The probability of success in phase III and prelaunch is 80%. a. b. C. d. What is the NPV? Is the extra $20 million investment worthwhile? Assuming that the probability of success in the phase III trials falls to 75%, recalculate the NPV with the extra 20 million investment. Is the extra $20 million investment worthwhile at this time? A candidate drug requires an $18 million investment for phase II clinical trials. If the trials are successful (44% probability), the company will learn the drug's scope of use and update its forecast of the drug's PV at commercial launch. The investment required for the phase III trials and prelaunch outlays is $130 million. The probability of success in phase III and prelaunch is 80%. Launch comes three years after the start of phase Ill if the drug is approved by the FDA. The probabilities of the upside, most likely, and downside outcomes are 25%, 50%, and 25%, respectively. If the phase III trials are successful, the manager will learn the commercial potential of the drug, which will depend on how widely it can be used. Suppose that the forecasted PV at launch depends on the scope of use allowed by the FDA. These are an upside outcome of NPV = $700 million if the drug can be widely used, a most likely case with NPV = $300 million, and a downside case of NPV = $100 million if the drug's scope is greatly restricted. We assume a risk-free rate of 4% and market risk premium of 7%. Assume FDA-approved pharmaceutical products have asset betas of 0.8 and the opportunity cost of capital is 9.6%. Accordingly, the NPV works out to $19 million. The R&D team has put forward a proposal to invest an extra $20 million in expanded phase II trials. Phase II takes two years. Th object is to prove that the drug can be administered by a simple inhaler rather than as a liquid. If successful, the scope of use is broadened and the upside PV increases to $1 billion. The probabilities of success are unchanged. Invest 18? Phase II Trials, 2 years 56% Learn potential PV Succeed 44% Fail PV=0 25% 50% 25% Phase Trials and prelaunch Invest 130? Yes, NPV = +295 Invest 130? Yes, NPV +52 Invest 130? STOP, PV = 0 20% 20% 20% years) Succeed 80% Fail, PV = 0 Succeed 80% Fail, PV=0 Succeed 80% Fail, PV = 0 Upside PV = 700 Most likely PV = 300 Downside PV = 100 FIGURE 10.7 A simplified decision tree for pharmaceutical R&D. A candidate drug requires an $18 million investment for phase Il clinical trials. If the trials are successful (44% probability), the company learns the drug's scope of use and updates its forecast of the drug's PV at commercial launch. The investment required for the phase III trials and prelaunch outlays is $130 million. The probability of success in phase III and prelaunch is 80%. a. b. C. d. What is the NPV? Is the extra $20 million investment worthwhile? Assuming that the probability of success in the phase III trials falls to 75%, recalculate the NPV with the extra 20 million investment. Is the extra $20 million investment worthwhile at this time?
Step by Step Solution
★★★★★
3.49 Rating (176 Votes )
There are 3 Steps involved in it
Step: 1
Original case NPV upside 130 0870010963 295 million NPV most likely 130 0830010963 52 million NPV do...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


