Question: A cascade of 3 isothermal continuous stirred tank reactors (CSTR) in series is used to carry out the following liquid-phase reaction, as show in
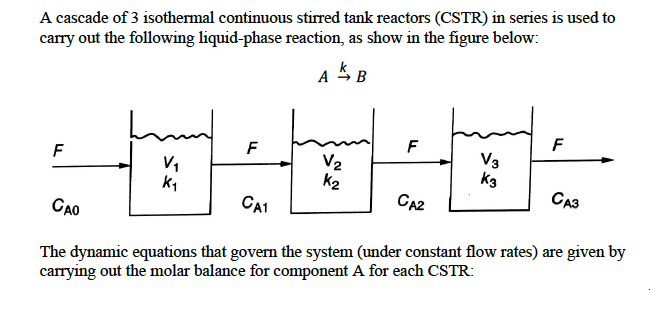
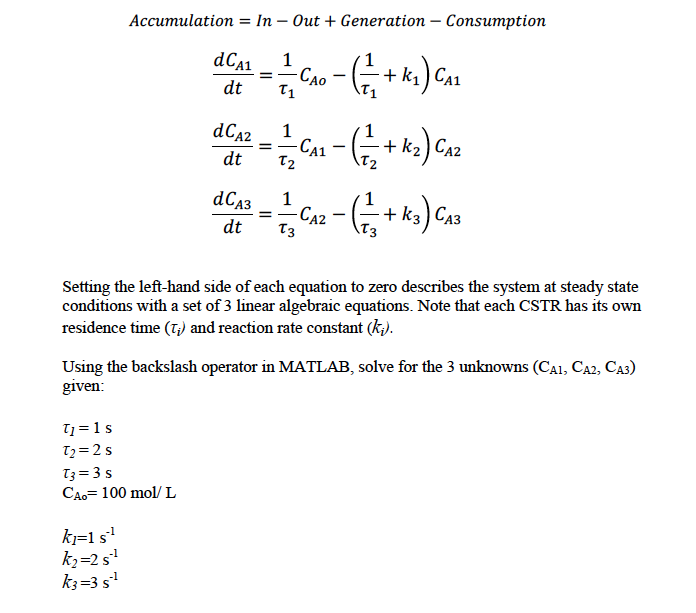
A cascade of 3 isothermal continuous stirred tank reactors (CSTR) in series is used to carry out the following liquid-phase reaction, as show in the figure below: F 20:0:0: CA1 F F CA2 V3 K3 F CA3 CAO The dynamic equations that govern the system (under constant flow rates) are given by carrying out the molar balance for component A for each CSTR: Accumulation = In-Out + Generation - Consumption dCA1 1 dt T1 == T=1 s T=2s T3 = 3 s CAo= 100 mol/L k=1 s k=2 s k3=3 s -CAO - ( = 1 + K) C CA1 dCA2 1 dt == - CA - (- == + K) CAZ T2 dCA3 1 == dt Setting the left-hand side of each equation to zero describes the system at steady state conditions with a set of 3 linear algebraic equations. Note that each CSTR has its own residence time (T;) and reaction rate constant (k.). -CA2 - ( = =+ K3) CA3 k3 T3 Using the backslash operator in MATLAB, solve for the 3 unknowns (CA1, CA2, CA3) given:
Step by Step Solution
3.29 Rating (149 Votes )
There are 3 Steps involved in it
To solve the problem we need to find the steadystate concentrations CA1 CA2 CA3 for the series of th... View full answer

Get step-by-step solutions from verified subject matter experts


