Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A change in temperature of the equilibrium system below increases the value of the equilibrium constant. The new state of equilibrium was established by a
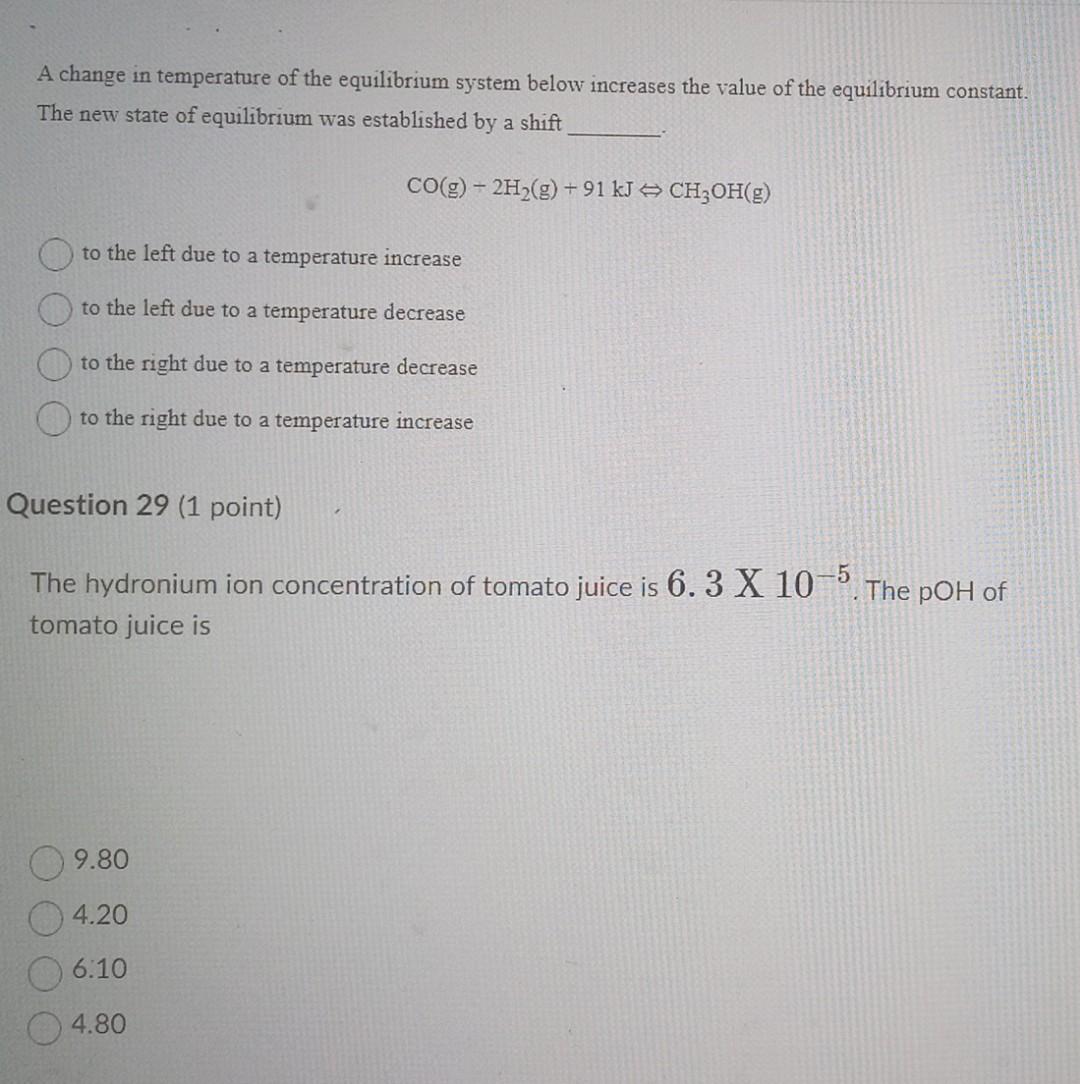
A change in temperature of the equilibrium system below increases the value of the equilibrium constant. The new state of equilibrium was established by a shift CO(g)2H2(g)+91kJCH3OH(g) to the left due to a temperature increase to the left due to a temperature decrease to the right due to a temperature decrease to the right due to a temperature increase Question 29 (1 point) The hydronium ion concentration of tomato juice is 6.3X105. The pOH of tomato juice is 9.80 4.20 6.10 4.80
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


