Question
A chemical company wants to produce 350 tons of ethylene oxide per day by reaction between ethylene and oxygen at a temperature of 463 K
A chemical company wants to produce 350 tons of ethylene oxide per day by reaction between ethylene and oxygen at a temperature of 463 K and 20 atm. You have been hired to do the calculations for the reactor design. the reaction is C2H4 + O2 C2H4O or symbolically A + B C and the following data was provided by the company:
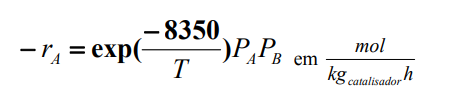
- The molar fraction of A in the feed can vary between 5 and 30%; - The molar fraction of B in the feed can vary between 5 and 20%; - You can use pure O2 or air (21% O2 and 79% N2); - The conversion is a maximum of 55%; - The volumetric flow rate in the feed can vary between 1.0105 and 1.5105 L/min; Make your best proposal* to the company, that is, present your project to production of ethylene oxide. You need to convince the company's directors and for that you need to show the calculations involved in the project. Make up your own mind if needed. * You must show in the proposal: Total catalyst mass, feed conditions (all molar flows), use or not of inert, number of tubes, conditions at the exit (all molar flows).
rA=exp(T8350)PAPBemkgcatalisadorhmolStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


