Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemical engineer investigating the conversion rate in the reaction of ethyl acetate with sodium hydroxide is conducting analysis through back titration. The solution used
A chemical engineer investigating the conversion rate in the reaction of ethyl acetate with
sodium hydroxide is conducting analysis through back titration. The solution used in titration
is caustic soda sodium hydroxide The concentrations of ethyl acetate and caustic
soda involved in the reaction are both moles. In the sample containers, there is a
phenolphthalein indicator and of hydrochloric acid.
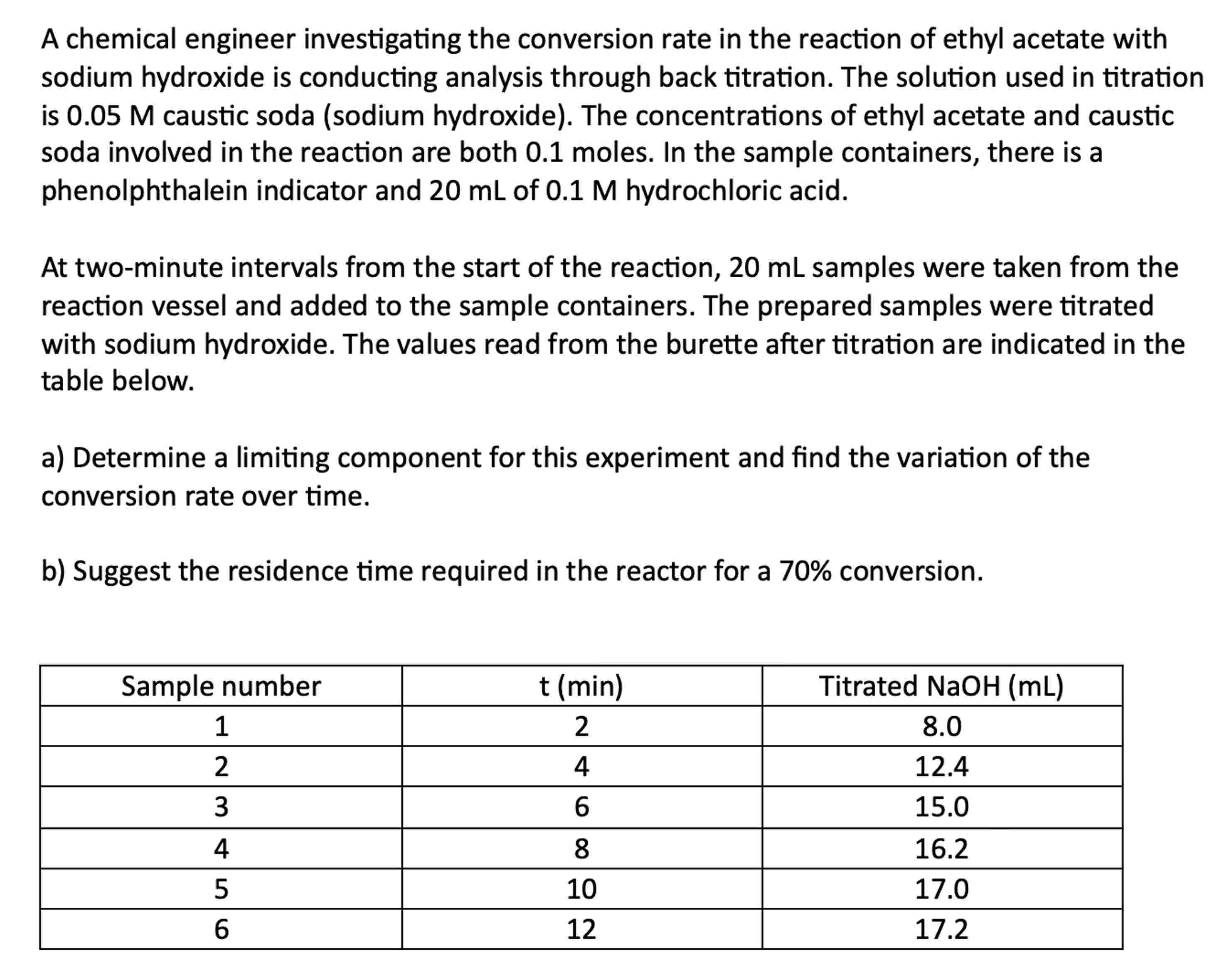
At twominute intervals from the start of the reaction, samples were taken from the
reaction vessel and added to the sample containers. The prepared samples were titrated
with sodium hydroxide. The values read from the burette after titration are indicated in the
table below.
a Determine a limiting component for this experiment and find the variation of the
conversion rate over time.
b Suggest the residence time required in the reactor for a conversion.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


