Answered step by step
Verified Expert Solution
Question
1 Approved Answer
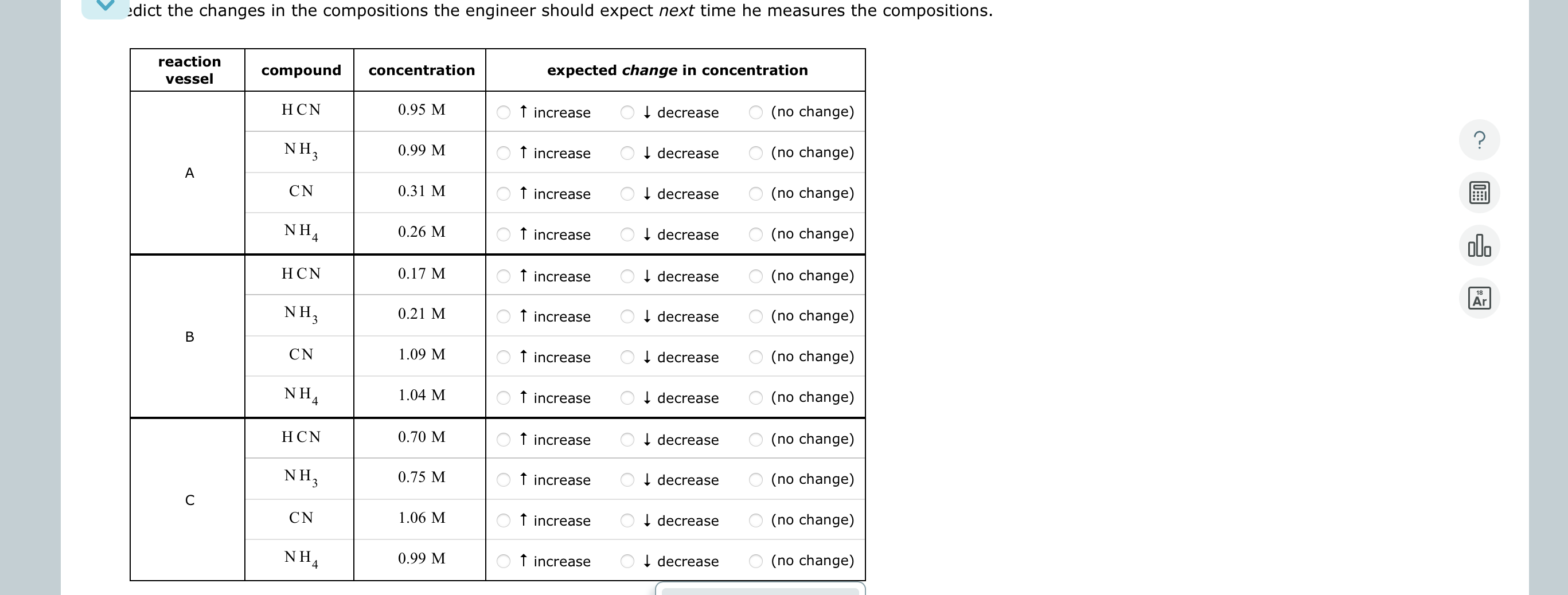
A chemical engineer is studying the following reaction: HCN ( aq ) + NH _ 3 - > CN ^ - ( aq ) +
A chemical engineer is studying the following reaction: HCNaqNH CNaqNHaq At the temperature the engineer picks, the equilibrium constant Kc for this reaction is The engineer charges fills four reaction vessels with hydrogen cyanide and ammonia, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below.
Predict the changes in the compositions the engineer should expect next time he measures the compositions. Please help! This is due soon
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


