Question
A chemical plant is emitting an air steam containing 30 mol% Contaminant Q. You are asked to design a tray tower that will use pure
A chemical plant is emitting an air steam containing 30 mol% Contaminant Q. You are asked to design a tray tower that will use pure water to reduce the Q levels to 3 mol%. The column is to operate at 1 atm and 20°C. The air flow rate is 200 kg air/h-m2 and the entering water rate is 8,000 kg/h-m2 ; these air and water flow rates are on a Q-free basis. Assume that water is non-volatile at these operating conditions and that the solubility of air in water at these conditions is negligible. The molecular weight of Q is 64 lb/lb-mol. a) For a 30% tray efficiency, determine the number of theoretical and actual stages required for this separation. b) One concern raised is that the temperature of the air stream could be much higher coming from the combustion plant. Discuss how this could impact your design estimate. c) What could be done with the exiting water stream containing the Q?
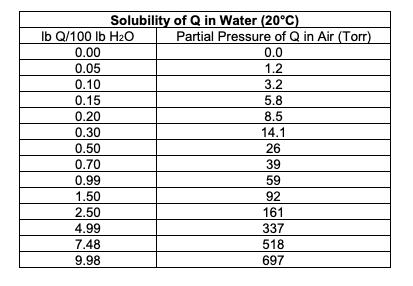
Solubility lb Q/100 lb HO 0.00 0.05 0.10 0.15 0.20 0.30 0.50 0.70 0.99 1.50 2.50 4.99 7.48 9.98 of Q in Water (20C) Partial Pressure of Q in Air (Torr) 0.0 1.2 3.2 5.8 8.5 14.1 26 39 59 92 161 337 518 697
Step by Step Solution
3.56 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
a The number of theoretical stages required for this separation is 15 The number of actual sta...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


