Answered step by step
Verified Expert Solution
Question
1 Approved Answer
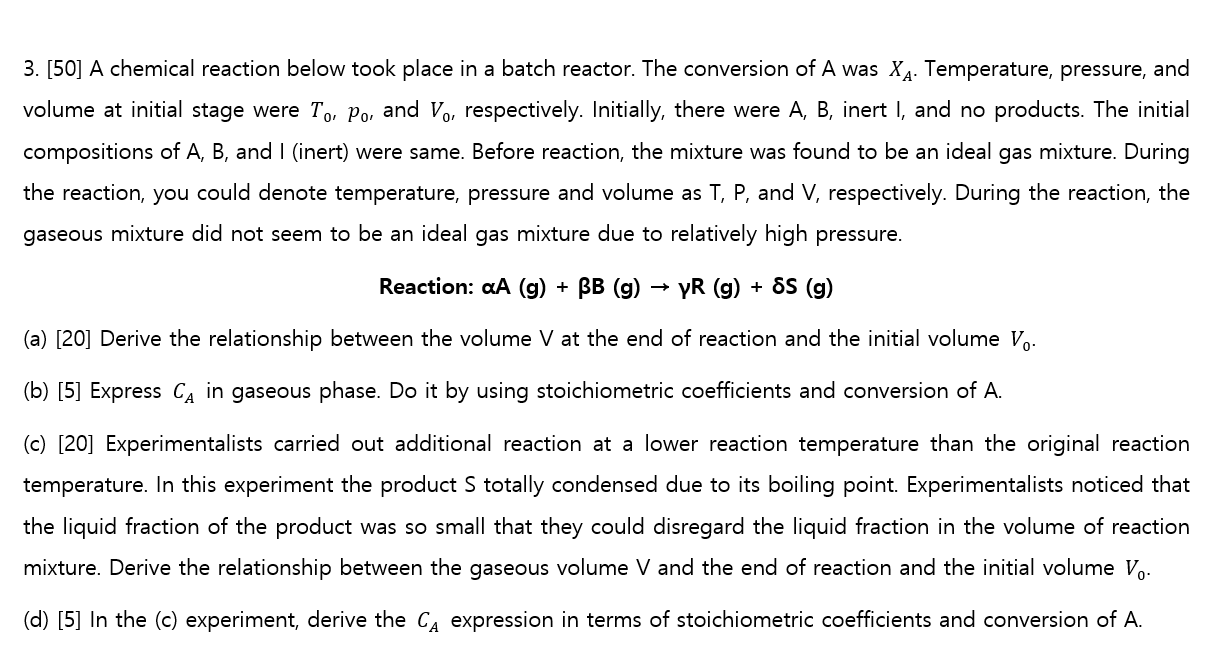
A chemical reaction below took place in a batch reactor. The conversion of A was x A . Temperature, pressure, and volume at initial stage
A chemical reaction below took place in a batch reactor. The conversion of A was Temperature, pressure, and
volume at initial stage were and respectively. Initially, there were inert I, and no products. The initial
compositions of and I inert were same. Before reaction, the mixture was found to be an ideal gas mixture. During
the reaction, you could denote temperature, pressure and volume as and respectively. During the reaction, the
gaseous mixture did not seem to be an ideal gas mixture due to relatively high pressure.
Reaction:
a Derive the relationship between the volume at the end of reaction and the initial volume
b Express in gaseous phase. Do it by using stoichiometric coefficients and conversion of
c Experimentalists carried out additional reaction at a lower reaction temperature than the original reaction
temperature. In this experiment the product totally condensed due to its boiling point. Experimentalists noticed that
the liquid fraction of the product was so small that they could disregard the liquid fraction in the volume of reaction
mixture. Derive the relationship between the gaseous volume and the end of reaction and the initial volume
d In the c experiment, derive the expression in terms of stoichiometric coefficients and conversion of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


